354
views
views

The international ISO 13485 certification standard that defines the requirements for the Quality Management system for the manufacturers, contract services, suppliers, distributors of the medical devices.
ISO 13485 Certification for Medical Devices
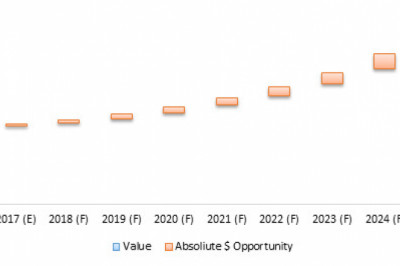
The ISO 13485 standard for the medical devices- Quality management system- Requirements for regulatory purpose, is one of the major bases for the regulatory compliance in the local and most of the international markets. ISO 13485 Certification is mandatory for the companies who are into the export of medical devices to other countries. The main objective of the standard is to bring harmony between the legal requirements and the management system being followed to manufacture or trade the medical devices.
Benefits of implementing ISO 13485:
- Expansion in national and international market Access
- Meet and regularly monitor Regulatory Requirements
- Gain a competitive edge
iso 13485 certification