views
Randomization and Trial Supply Management (RTSM) Market Analysis and Size
Randomization and trial supply management is abbreviated as RTSM. It's a technology solution that's utilised in clinical studies to manage drug distribution and control patient randomization. Randomization is an experimental control strategy that eliminates selection and unintentional bias.
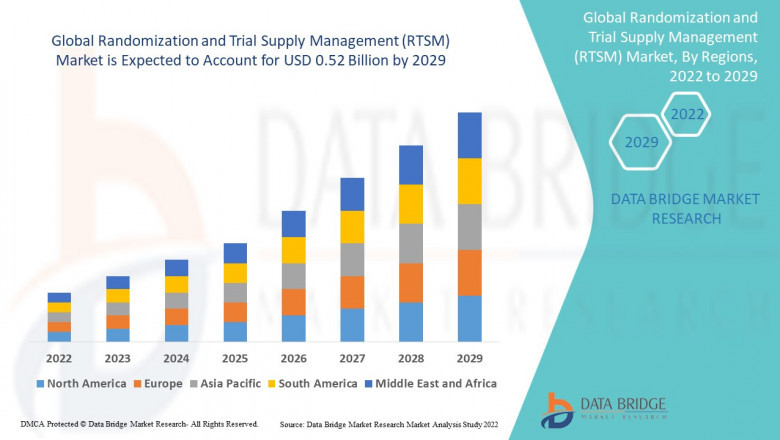
Data Bridge Market Research analyses that the randomization and trial supply management (RTSM) market which was USD 0.17 billion in 2021, would rocket up to USD 0.52 billion by 2029, and is expected to undergo a CAGR of 15 % during the forecast period 2022 to 2029. In addition to the market insights such as market value, growth rate, market segments, geographical coverage, market players, and market scenario, the market report curated by the Data Bridge Market Research team also includes in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Clinical studies use telephone and interactive voice response (IVR) technologies to manage randomization, study medication administration, and inventories using randomization and trial supply management (RTSM) systems.
Get PDF Sample on :- https://www.databridgemarketresearch.com/request-a-sample/?dbmr=global-randomization-and-trial-supply-management-rtsm-market
Randomization and Trial Supply Management (RTSM) Market Dynamics
Drivers
· Rise in adoption of novel software solutions
Increasing adoption of novel software solutions, rising government funding for clinical trials, the introduction of e-clinical solutions to improve data standardisation, and rising pharmaceutical company expenditure for drug development pipeline allocation are some of the factors that will likely accelerate the growth of the randomization and trial supply management (RTSM) market, in the forecast period of 2022-2029.
· increasing number of clinical trials
The increasing number of clinical trials in developing economies, as well as the outsourcing of clinical trial operations by many sectors, would enhance numerous opportunities for the randomization and trial supply management (RTSM) market throughout the forecast period.
Opportunities
· Rising awareness among the patients regarding the prevailing advantages of management solutions will help in boosting lucrative opportunities in the growth of the market.
Global Randomization and Trial Supply Management (RTSM) Market Scope
The randomization and trial supply management (RTSM) market is segmented on the basis of delivery mode, clinical trial phase and end-user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Delivery Mode
· Web-Hosted (On-Demand) Solutions
· Licensed Enterprise (On-Premise) Solutions
· Cloud-Based (SaaS) Solutions
Clinical Trial Phase
· Phase I Clinical Trials
· Phase II Clinical Trials
· Phase III Clinical Trials
· Phase IV Clinical Trials
End-User
· Pharmaceutical
· Biopharmaceutical Companies
· Contract Research Organizations
· Consulting Service Companies
· Medical Device Manufacturers
· Hospitals
· Academic Research Institutes
Get Toc detail of the Report:- https://www.databridgemarketresearch.com/toc/?dbmr=global-randomization-and-trial-supply-management-rtsm-market
Randomization and Trial Supply Management (RTSM) Market Regional Analysis/Insights
The randomization and trial supply management (RTSM) market is analysed and market size insights and trends are provided by country, delivery mode, clinical trial phase and end-user as referenced above.
The countries covered in the randomization and trial supply management (RTSM) market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America dominates the randomization and trial supply management (RTSM) market due to the growing occurrences of lifestyle diseases along with growing population and prevalence of improved infrastructure.
Asia-Pacific is expected to grow at the highest growth rate in the forecast period of 2022 to 2029 due to the prevalence of targeted diseases along with rising number of clinical trials.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Some of the major players operating in the randomization and trial supply management (RTSM) market are:
· MEDICAL Information Technology Inc. (U.S)
· SAP (Germany)
· CPSI (U.S)
· Meta (U.S)
· Elinext (U.S)
· EPIC Systems Corporation (U.S)
· INFOR (U.S)
· Cognizant (U.S)
· Oracle (U.S)
· Jag products LLC (U.S)
· Allscripts Healthcare LLC (U.S)
· Optum Inc. (U.S)
· Cerner Corporation (U.S)
· Change Healthcare (U.S)
· Koninklijke Philips N.V. (Netherlands)
· athenahealth (U.S)
· eClinicalWorks (U.S)
Browse in-depth Research Report @ https://www.databridgemarketresearch.com/reports/global randomization-and-trial-supply-management-rtsm-market
About us: -
An absolute way to forecast what future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data bridge endeavours to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in pune.
Data bridge market research has over 500 analysts working in different industries. We have catered more than 40% of the fortune 500 companies globally and ha bridge market research has over 500 analysts working in different industries. We have catered more than 40% of the fortune 500 companies globally and have a network of more than 5000+ clientele around the globe. Data bridge adepts in creating satisfied clients who reckon upon our services and rely on our hard work with certitude. We are content with our glorious 99.9 % client satisfying rate.
Contact us: -
Data bridge market research
Us: +1 888 387 2818
Email: - corporatesales@databridgemarketresearch.com