views

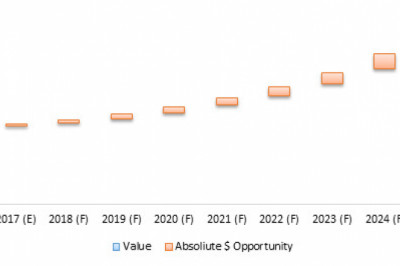
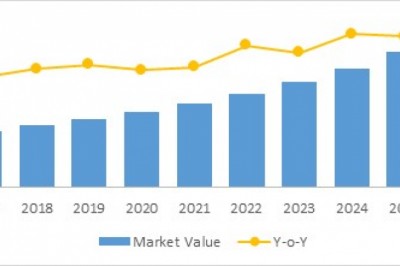
Radioligand therapeutics, also known as radiopharmaceuticals, which show antitumor effects, has seen rapid development over the past decade. Although some therapies are already approved for human use, many more are in the clinical trial and will enter clinical practice in the next 2-7 years, potentially introducing new therapeutic choices for patients. Despite this innovation, several challenges remain, including supply chain, logistics, regulatory issues, and education and training. The current market for radioligand therapy is majorly dominated by manufacturers such as Novartis AG, Pfizer Inc, Johnson Johnson Services, Inc, Telix Pharmaceuticals, Lantheus Holdings Inc., (Progenics Pharmaceuticals), Molecular Partners AG, Clovis Oncology Inc., Fusion Pharma, Point Biopharma, Precirix, ITM Isotope Technologies Munich SE, Curium Pharma, and RadioMedix. The global radioligand therapy market was valued at $9,754.9 million in 2020 and is expected to reach $16,658.4 million by 2031, witnessing a CAGR of 4.67% during the forecast period 2021-2031.

COVID-19 Impact on Global Radioligand Therapy Market
The global spread of COVID-19, caused by the novel coronavirus SARS-CoV-2, has resulted in a continuing pandemic threat to global health. Nuclear medicine techniques can be used for functional imaging of pathophysiological processes at the cellular level and for treatment approaches based on targeted delivery of therapeutic radionuclides. Ongoing development of radiolabeling methods has significantly improved the accessibility of radiopharmaceutical products for targeted radionuclide therapy, but their use for biosafety threats such as SARS-CoV-2 is restricted by the contagious nature of these agents.
Moreover, radioligand therapies rely on complex supply chains and advanced logistics. The lockdowns imposed by most countries and the closure of borders have generated shortages of radionuclides and other essential raw material supplies in many countries
Competitive Landscape
The global radioligand therapy market comprises well-established and newly emerging companies. Several companies are attempting to sustain their position in the market by launching new products and raising funds to develop new products for the innovation in radioligand therapy from different sample types. Key market players of the global radioligand therapy market witnessed product launch, product approval and business funding, and synergistic activities majorly during the period January 2018-February 2022. The inclination of companies toward product launches and product approval suggests that the companies are involved in continuously bringing new products to the market to radioligand therapy, which is primarily attributed to a rising prevalence of cancer, rising clinical research activity, and rising key players' initiatives are driving the market.
Global Radioligand Therapy Market Segmentation
• Product (Approved Products and Potential Pipeline)
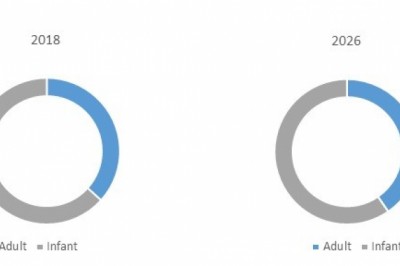
• Indication (Prostate Cancer, Neuroendocrine Tumor (NETs), and Others)
• Biomarker (Prostate-Specific Membrane Antigen, Ki 67 Expression and Grading, Cytochrome P450 17A1 Inhibitor)
Regional Segmentation
• North America - U.S., Canada
• Europe - Germany, U.K., France, Italy, Spain, and Rest-of-Europe
• Asia-Pacific - Japan, South Korea, Australia, and Rest-of-Asia-Pacific
• Rest-of-the-World
Market Growth Drivers
• Radioligand Therapy Demand Inclined by Rising Prevalence of Cancer
• Strategic Initiatives by Key Market Players
• Rise in Clinical Research Activity
Market Challenges
• High Cost Associated with Treatment and Complex Reimbursement Processes
• Increased Competition from Generics
Market Opportunities
• Expanding Radiopharmaceutical Coverage
• Role of Radioligand in Drug Discovery
Key Companies Profiled
Johnson Johnson Services, Inc., Pfizer Inc., Amneal Pharmaceuticals LLC, Novartis International AG, POINT Biopharma Global Inc, Fusion Pharma, Clovis Oncology, Telix Pharmaceuticals, Lantheus Holdings, Inc. (Progenics Pharmaceuticals), Bayer AG, Molecular Partners, ITM Isotope Technologies Munich SE, Curium Pharma, Precirix, Radio Medix
Key Questions Answered in this Report:
• How is radioligand therapy revolutionizing oncology?
• What are the major market drivers, challenges, and opportunities in the global radioligand therapy market?
• What are the underlying structures resulting in the emerging trends within the global radioligand therapy market?
• How is the COVID-19 pandemic impacting the global radioligand therapy ecosystem?
• What are the key development strategies that the major players are implementing in order to sustain themselves in the competitive market?
• What are the key regulatory implications in developed and developing regions pertaining to the use of radioligand-targeted therapies?
• What are the potential entry barriers expected to be faced by the companies willing to enter a particular region?
• How is each market segment expected to grow during the forecast period 2021-2031, and what is the anticipated revenue to be generated by each segment? Following are the segments:
o Products (Approved Products and Potential Pipeline)
o Indication (Prostate Cancer, Neuroendocrine Tumor (NETs), and Others)
o Biomarker (Prostate-Specific Membrane Antigen, Ki 67 Expression and Grading, Cytochrome P450 17A1 Inhibitor)
o Region (North America, Europe, Asia-Pacific, and Rest-of-the-World)
• What are the growth opportunities for the radioligand therapy companies in the region of their operation?
• Who are the leading players with significant offerings in the global radioligand therapy market?
• Which companies are anticipated to be highly disruptive in the future, and why?
Request Sample - https://bisresearch.com/requestsample?id=1280type=download
The scope of the report exclusively covers manufacturers offering proprietary radioligand therapy for target indications such as prostate cancer and neuroendocrine tumors. The study considers products based on various radioisotopes underlying applications in oncology to detect biomarkers and treatment of cancers. Moreover, the study considers the generics sales of Zytiga.