views
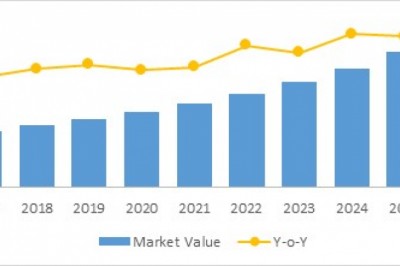
The U.S.Transthyretin Amyloidosis Treatment Market by Drug (Tafamidis, Diflunisal,Patisiran, Inotersen, and Others), By Disease Type (Hereditary TransthyretinAmyloidosis (Polyneuropathy, Cardiomyopathy, Mixed), and Wild Type), and ByDistribution Channel (Hospital Pharmacies, Retail Pharmacies, and OnlinePharmacies) is expected to be valued at US$ 36.9 million in 2018 and isprojected to exhibit a CAGR of 52.4% over the forecast period (2018–2026).Unavailability of any alternative treatment options, expected drug launches in2018, and rapid uptake of the newly available therapies are expected to driveU.S. transthyretin amyloidosis treatment market growth.
In 2017, the U.S. Food and DrugAdministration (FDA) granted breakthrough therapy designation (BTD) toPatisiran for the treatment of hereditary ATTR (hATTR) amyloidosis withpolyneuropathy. Moreover, in November 2017, Alnylam Pharmaceuticals, Inc.initiated a New Drug Application (NDA) for Patisiran and the company announcedU.S. FDA acceptance of NDA and priority review status for Patisiran in February2018.
Manufacturers are actively focusedon developing new therapies for the treatment transthyretin amyloidosis. Thereis no treatment available in the U.S. for transthyretin amyloidosis, which isexpected to create lucrative opportunities for manufacturers in the U.S.transthyretin amyloidosis market over the forecast period.
* The sample copy includes: Report Summary, Table ofContents, Segmentation, Competitive Landscape, Report Structure, Methodology.
Request a sample copyof this report: https://www.coherentmarketinsights.com/insight/request-sample/1919
In January 2018, the new drugapplication (NDA) of Inotersen was accepted for priority review by the U.S.Food and Drug Administration and a date of July 2018 was set for PrescriptionDrug User Fee Act (PDUFA), which was later postponed to October 6, 2018.Inotersen launch is expected to further boost the transthyretin amyloidosistreatment market growth.
Browse 22 Market Data Tables and25 Figures spread through 116 Pages and in-depth TOC on U.S. TransthyretinAmyloidosis Treatment Market by Drug (Tafamidis, Diflunisal, Patisiran,Inotersen, and Others), By Disease Type (Hereditary Transthyretin Amyloidosis(Polyneuropathy, Cardiomyopathy, Mixed), and Wild Type), and By DistributionChannel (Hospital Pharmacies, Retail Pharmacies, and Online Pharmacies) -Forecast to 2026
Key players in the market arefocused on collaborations, acquisitions, and partnerships, in order to launchtheir new therapies into the market. For instance, AKCEA-TTR-LRX, a LigandConjugated Antisense (LICA), is being developed by Akcea Therapeutics(affiliate of Ionis Pharmaceuticals), and is expected to enter clinical trialsin 2018 for the treatment of all forms of ATTR amyloidosis.
In March 2018, IonisPharmaceuticals completed a previously announced transaction with its affiliateAkcea Therapeutics that was related to commercialization rights of Inotersenand AKCEA-TTR-LRX globally (given to Akcea Therapeutics). AlnylamPharmaceuticals, Inc. developed the drug in collaboration with Sanofi and thedeal related to Patisiran was restructured inJanuary 2018.
Browse ResearchReport: https://www.coherentmarketinsights.com/market-insight/us-transthyretin-amyloidosis-treatment-market-1919
Under the new deal, Alnylamregained rights for global development and commercialization of its investigationalRNAi therapeutics such as Patisiran and ALN-TTRsc02, whereas Sanofi assumedfull responsibility for the development and commercialization of Fitusiran(Hemophilia A and B).
Key Takeaways of the U.S.Transthyretin Amyloidosis Treatment Market:
The U.S. transthyretinamyloidosis treatment market is expected to exhibit a CAGR of 52.4% over theforecast period (2018–2026), owing to product launches by manufacturers such asAlnylam Pharmaceuticals, Inc., and Ionis Therapeutics
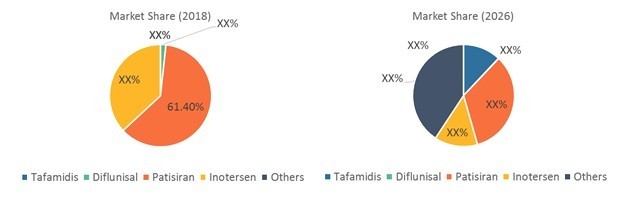
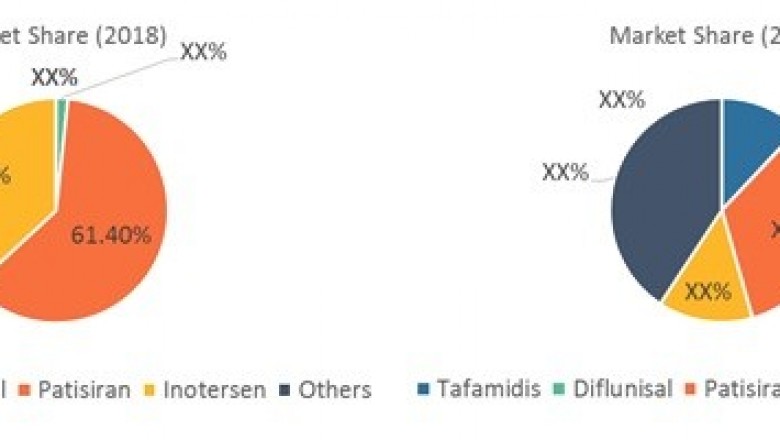
Patisiran (Alnylam Pharmaceuticals,Inc.) is currently ahead of its competitors in terms of approval and subsequentcommercialization, and Alnylam may benefit from the first-mover advantage
Therapies in the pipeline belongto the class of gene therapies (RNAi therapeutics and antisense drugs) whichhave the potential to cure the underlying cause of the disease
Major players operating in theU.S. transthyretin amyloidosis treatment market include AlnylamPharmaceuticals, Inc., Pfizer, Inc., Prothena Corporation Plc, GlaxoSmithKlinePlc, Ionis Pharmaceuticals, Inc., Eidos Therapeutics, and SOM InnovationBiotech, S.L.
Buy-Now this researchreport: https://www.coherentmarketinsights.com/insight/buy-now/1919
AboutCoherent Market Insights:
CoherentMarket Insights is a prominent market research and consulting firm offeringaction-ready syndicated research reports, custom market analysis, consultingservices, and competitive analysis through various recommendations related toemerging market trends, technologies, and potential absolute dollar opportunity.
ContactUs:
mailto:sales@coherentmarketinsights.com
U.S.Office:
Name: Mr. Shah
CoherentMarket Insights 1001 4th Ave,
# 3200Seattle, WA 98154, U.S.
US : +1-206-701-6702
UK : +44-020-8133-4027
JAPAN: +050-5539-1737