views
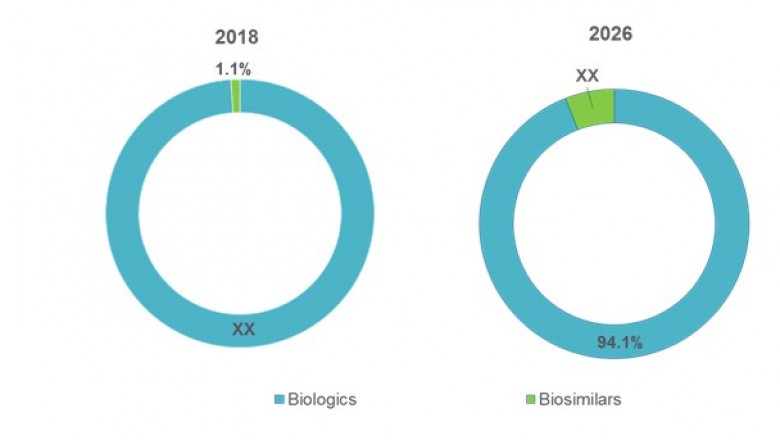
The U.S.Non-oncology Biopharmaceuticals Market, by Product Type (Biologics andBiosimilars), by Drug Class (Monoclonal Antibody, Enzyme Replacement Therapy,Erythropoietin, Interferon, Colony-stimulating Factor, Botulinum Toxin, FusionProtein, Vaccines, Human Recombinant Insulin, Blood Factors, Human GrowthHormone, and Others), and by Disease Indication (Diabetes, RheumatoidArthritis, Psoriatic Arthritis, Multiple Sclerosis, Hemophilia, Anemia,Age-related Macular Degeneration, Hepatitis B, Cystic Fibrosis, Osteoporosis,Crohn’s Disease, Ulcerative Colitis, Psoriasis, Ankylosing Spondylitis, andOthers (Cardiac Diseases and Others)), was valued at US$ 116.0 Bn in 2018 andis projected to exhibit a CAGR of 10.2% during the forecast period (2018–2026).
Factors such as increasing launchand approval of novel biopharmaceuticals and robust pipeline ofbiopharmaceutical products in late phase of clinical trial is expected tosignificantly drive the U.S. non-oncology biopharmaceuticals market growth. Forinstance, in October 2018, Leadiant Biosciences, Inc. received the U.S. Foodand Drug Administration (FDA) approval for its Revcov (elapegademase-lvlr)injection in the U.S. Revcovi is a new enzyme replacement therapy (ERT) for thetreatment of adenosine deaminase severe combined immune deficiency (ADA-SCID)in pediatric and adult patients. Furthermore, key players are involved indevelopment and launch of novel biopharmaceuticals for new indications. Forinstance, in May 2018, Novartis announced that the U.S. FDA approved Aimovig(erenumab) for the preventive treatment of migraine in adults. Aimovig was thefirst human monoclonal antibody approved for treatment of migraine. InSeptember 2018, Eli Lilly and Company, and Teva Pharmaceutical Industries Ltd.also received the U.S. FDA approval for Emgality (galcanezumab-gnlm) and AJOVY(fremanezumab-vfrm), respectively for the preventive treatment of migraine inadults. Launch of novel drugs for new indications is expected to support theU.S. non-oncology biopharmaceuticals market growth over the forecast period.
* The sample copy includes: Report Summary, Table ofContents, Segmentation, Competitive Landscape, Report Structure, Methodology.
Request a sample copyof this report: https://www.coherentmarketinsights.com/insight/request-sample/2575
Browse 35 Market Data Tables and38 Figures spread through 224 Pages and in-depth TOC on U.S. Non-oncologyBiopharmaceuticals Market, by Product Type (Biologics and Biosimilars), by DrugClass (Monoclonal Antibody, Enzyme Replacement Therapy, Erythropoietin,Interferon, Colony-stimulating Factor, Botulinum Toxin, Fusion Protein,Vaccines, Human Recombinant Insulin, Blood Factors, Human Growth Hormone, andOthers), and by Disease Indication (Diabetes, Rheumatoid Arthritis, PsoriaticArthritis, Multiple Sclerosis, Hemophilia, Anemia, Age-related MacularDegeneration, Hepatitis B, Cystic Fibrosis, Osteoporosis, Crohn’s Disease,Ulcerative Colitis, Psoriasis, Ankylosing Spondylitis, and Others (CardiacDiseases and Others)) - U.S. Forecast to 2026.
Key players in the market areinvolved in strategic merger, collaboration, acquisition, and partnership forthe development of novel biopharmaceuticals to expand its portfolio withstrategic acquisition of any company. For instance, in October 2018, TevaPharmaceutical Industries Ltd. and Celltrion, Inc. entered into an exclusivepartnership to commercialize two of Celltrion’s mAb biosimilar candidates inthe U.S. and Canada. In 2016, AbbVie Inc. acquired all rights from BoehringerIngelheim (BI) for Risankizumab (BI 655066). Risankizumab is an anti-IL-23monoclonal biologic antibody for psoriasis. Company is also evaluating theproduct for other indications such as Crohn’s disease, asthma, and psoriaticarthritis. Currently, it is in clinical phase 3. Furthermore, patent loss ofblockbuster drugs such as Humira and launch of their biosimilars at low pricesis expected to be a major factor negatively affecting the revenue generated byblockbuster drugs, which in turn is expected to negatively affect the overallU.S non-oncology biopharmaceutical market size and growth over the forecastperiod. For instance, in the recent past, various players such as Sandoz Inc.,Boehringer Ingelheim GmbH, and Amgen Inc., received the U.S. FDA approval forbiosimilar versions of AbbVie Inc.’s blockbuster drug, Humira (adalimumab) inthe U.S. However, most of these companies have reached a settlement agreementwith AbbVie Inc. to delay launch of Humira biosimilar up to 2023.
Browse ResearchReport: https://www.coherentmarketinsights.com/market-insight/u-s-non-oncology-biopharmaceuticals-market-2575
Key Takeaways of the U.S.Non-oncology Biopharmaceuticals Market:
The U.S. non-oncologybiopharmaceuticals market is expected to exhibit a CAGR of 10.2% during theforecast period (2018 – 2026), owing to increasing product launches andapprovals, and robust pipeline of novel U.S. non-oncology biopharmaceuticals
The U.S. is the most lucrativeeconomy for monoclonal antibodies, as most of the key players such as AbbVieInc., Roche Holding AG, and Merck & Co. generate major revenue of theirbiological drugs from the U.S.
In the recent past, variousblockbuster biologics such as Humira and Remicade lost patent in the U.S.market. Furthermore, various other high revenue generating biologics areexpected to lose their patents in the near future. Loss of patents in the U.S. marketoffers lucrative opportunity to other players for development of biosimilars.
Key players in the market havelucrative opportunities to develop novel and innovative therapies for variousrare diseases and target the underserved patients. Acts such as the Orphan DrugAct also supports in creating financial incentives for companies to develop newdrugs for rare diseases.
Major players operating in theU.S. non-oncology biopharmaceuticals market include Sanofi S.A., Pfizer, Inc.,Johnson & Johnson, Novartis International AG, Amgen, Inc., Eli Lilly andCompany, AbbVie Inc., Bristol - Myers Squibb Company, F. Hoffmann-La Roche AG,Novo Nordisk A/S, GlaxoSmithKline plc., UCB Pharma, Teva PharmaceuticalIndustries Ltd., Takeda Pharmaceutical Company Ltd, AstraZeneca Plc, MylanN.V., LEO Pharma A/S, Boehringer Ingelheim GmbH, Alexion Pharmaceuticals Inc.,Merck & Co., Inc. Elusys Therapeutics, Inc., Swedish Orphan Biovitrum AB,Samsung Bioepis NL B.V., Biogen Inc., and Theratechnologies Inc.
Buy-Now this researchreport: https://www.coherentmarketinsights.com/insight/buy-now/2575
AboutCoherent Market Insights:
CoherentMarket Insights is a prominent market research and consulting firm offeringaction-ready syndicated research reports, custom market analysis, consultingservices, and competitive analysis through various recommendations related toemerging market trends, technologies, and potential absolute dollaropportunity.
Contact Us:
mailto:sales@coherentmarketinsights.com
U.S.Office:
Name: Mr. Shah
CoherentMarket Insights 1001 4th Ave,
# 3200Seattle, WA 98154, U.S.
US : +1-206-701-6702
UK : +44-020-8133-4027
JAPAN : +050-5539-1737