views
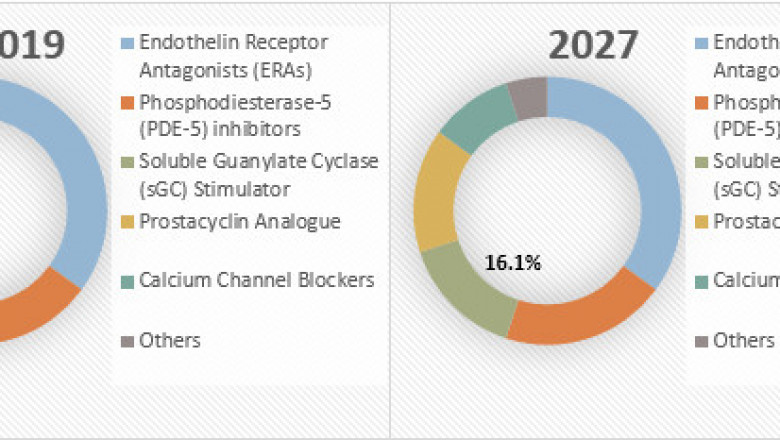
Global PulmonaryArterial Hypertension Market, by Drug Type (Endothelin Receptor Antagonists(ERAs), Phosphodiesterase-5 (PDE-5) Inhibitors, Soluble Guanylate Cyclase (sGC)Stimulators, Prostacyclin Analogue, Calcium Channel Blockers, and Others), byRoute of Administration (Oral, Inhaled, Intravenous, and Subcutaneous), by DistributionChannel (Hospital Pharmacies, Retail Pharmacies, and Online Pharmacies), and byRegion (North America, Latin America, Europe, Asia Pacific, Middle East, andAfrica) was valued at US$ 5,821.90 million in 2018, and is expected to exhibita CAGR of 5.9%, over the forecast period (2019-2027), as highlighted in a newreport published by Coherent Market Insights.
Increasing incidence of chroniclung or heart problems are expected to drive growth of the pulmonary arterialhypertension market over the forecast period. For instance, according to theCenters for Disease Control and Prevention (CDC), in 2014, in the U.S. anestimated prevalence of one or two cases of pulmonary hypertension occurs inevery 1 million U.S. population. Centers for Disease Control and Prevention(CDC), also reports that idiopathic pulmonary hypertension exist about 40.0% ofall the cases, whereas the prevalence of the disease among patients withsystemic sclerosis is about 10.0%, with sickle cell disease is about 3.0%, andamong HIV patients is 0.5%. Furthermore, the Centers for Disease Control andPrevention (CDC), states that the disease is 2-4 times more frequent amongwomen than men, which majorly diagnosed at the age of around 45 years old.
Major players are focused on druglaunches and regulatory approvals, which is expected to propel the marketgrowth over the forecast period. For instance, in September 2019, UnitedTherapeutics received the New Drug Application (NDA) approval by the U.S. Foodand Drug Administration (FDA), for the drug, Trevyent. Trevyent is a drugdevice combination product, which comprise PatchPump technology that enablestreprostilin administration by subcutaneous route. Moreover, in October 2019,United Therapeutics received an approval from the U.S. Food and DrugAdministration (FDA) for Orenitram, treprostilin tablets. The tablets areexpected to delay the progression of pulmonary arterial hypertension disease.
* The sample copy includes: Report Summary, Table ofContents, Segmentation, Competitive Landscape, Report Structure, Methodology.
Request a sample copyof this report: https://www.coherentmarketinsights.com/insight/request-sample/203
Browse 39 Market Data Tables and25 Figures spread through 163 pages and in-depth TOC on 'Pulmonary ArterialHypertension Market’- global forecast to 2027, by Drug Type (EndothelinReceptor Antagonists (ERAs), Phosphodiesterase-5 (PDE-5) Inhibitors, SolubleGuanylate Cyclase (sGC) Stimulators, Prostacyclin Analogue, Calcium ChannelBlockers, and Others), by Route of Administration (Oral, Inhaled, Intravenous,and Subcutaneous), by Distribution Channel (Hospital Pharmacies, RetailPharmacies, and Online Pharmacies), and by Region (North America, LatinAmerica, Europe, Asia Pacific, Middle East, and Africa)'
North America is expected to holda dominant position in the pulmonary arterial hypertension market, owing torising cases of pulmonary arterial hypertension and ongoing development andregulatory approvals for the associated drugs, which in turn is expected tofuel growth of the regional market over the forecast period. For instance,according to the CVS Health, the article published in April 2018 reports thatpulmonary arterial hypertension is considered as a deadly disease, whichaffects between 10,000 and 20,000 people in the U.S. and it affects womenmostly. According to the same source, pulmonary arterial hypertension is aprogressive condition, if remained untreated, around 70% of patients maysurvive for a year after diagnosis and only about one-third make it to 5 years.
Moreover, several major playersin the market are focusing on developing and gaining regulatory approvals ofthe product, which is expected to facilitate growth of the market over theforecast period. For instance, in March 2019, the U.S. Food & Drug Administrationapproved the very first generics drug for pulmonary arterial hypertension(PAH), Ambrisentan (Letairis).
Browse ResearchReport: https://www.coherentmarketinsights.com/market-insight/pulmonary-arterial-hypertension-pah-market-203
Key Takeaways of the PulmonaryArterial Hypertension Market:
The global pulmonary arterialhypertension market is expected to exhibit a CAGR of 5.9% over the forecastperiod, owing to rising cases that leads to pulmonary arterial hypertensionwhich includes, congestive heart failure, pulmonary embolism, and others. Forinstance, according to the American College of Cardiology Foundation publishedan article Heart Disease and Stroke Statistics-2019, a report from the AmericanHeart Association, which states that around 6.2 million U.S. adults had heartfailure (HF) in 2013-2016, whereas, in 2014, atrial fibrillation and pulmonaryembolism was the principal diagnosis in approximately 454,000 and 178,000respectively in U.S. hospitalizations.
Among drug type, endothelinreceptor antagonists (ERAs) segment held a dominant position in the pulmonaryarterial hypertension market in 2018, owing to the FDA approvals, which isexpected to propel the market growth. For instance, in September 2017, the U.S.Food & Drug Administration (FDA) approved Tracleer (bosentan) for thetreatment of pediatric pulmonary arterial hypertension (PAH). This drug is approvedfor the pediatric patients aged 3 years and older with idiopathic or congenitalPAH to improve pulmonary vascular resistance (PVR).
Companies operating in the globalpulmonary arterial hypertension market include United Therapeutics Corporation,GlaxoSmithKline plc, Novartis AG, AstraZeneca, Bayer AG, Merck KGaA, PfizerInc., Gilead Sciences, Inc., Actelion Pharmaceuticals Ltd, ArenaPharmaceuticals, and Daiichi Sankyo Company, Limited
Buy-Now this researchreport: https://www.coherentmarketinsights.com/insight/buy-now/203
AboutCoherent Market Insights:
CoherentMarket Insights is a prominent market research and consulting firm offeringaction-ready syndicated research reports, custom market analysis, consulting services,and competitive analysis through various recommendations related to emergingmarket trends, technologies, and potential absolute dollar opportunity.
ContactUs:
mailto:sales@coherentmarketinsights.com
U.S.Office:
Name: Mr. Shah
CoherentMarket Insights 1001 4th Ave,
# 3200Seattle, WA 98154, U.S.
US : +1-206-701-6702
UK : +44-020-8133-4027
JAPAN : +050-5539-1737












