views
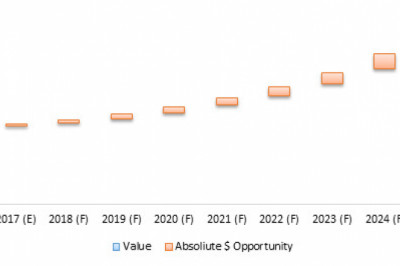
Global PrimarySclerosing Cholangitis Market, by Drug (BT1023, GS-9674, NGM282, OCA,LUM001, and Others) and by Region (North America, Latin America, Europe, AsiaPacific, Middle East, and Africa) has potential pipeline products and is estimatedto reach US$ 140.4 Mn by 2023.
Pharmaceutical companies arefocusing on enhancing research and development for introducing noveltherapeutics to address unmet patient needs. For instance, the discovery anddevelopment of siRNA (small interfering RNA) therapeutics by Sirnaomics, Inc.,with dual-target properties and polypeptide nanoparticle (PNP)-enhanceddelivery that directly controls the fibrotic and inflammatory activity isexpected to have wide applications in many inflammatory and fibrotic diseases.
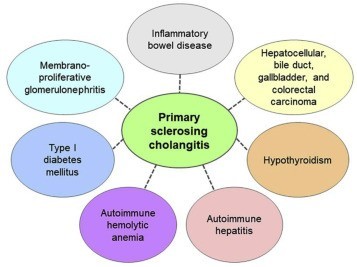
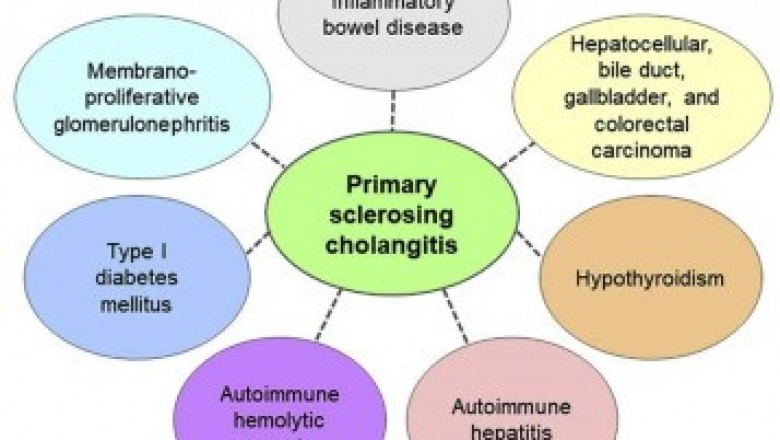
The support from the public andprivate organizations such as PSC Support Organization and NationalOrganization for Rare Disorders serves as the hub of rare disease community,providing assistance programs for patients and advancing clinical trials bygranting funds for advanced research programs. The UK PSC Support Organizationgranted US$ 20,000 to the University of Birmingham for supporting the researchand investigation of Vascular Adhesion Protein (VAP-1) in primary sclerosingcholangitis (PSC). Also, in 2017, the Falk Foundation funded a Europeanmulti-center phase II study led by the Medical University of Vienna incollaboration with the Medical University of Graz and the Medical University ofHannover, to treat primary sclerosing cholangitis.
Browse through 134 Pages and anin-depth TOC on Primary Sclerosing Cholangitis Market, by Drug (BT1023,GS-9674, NGM282, OCA, LUM001, and Others) and by Region (North America, LatinAmerica, Europe, Asia Pacific, Middle East, and Africa).
* The sample copy includes: Report Summary, Table ofContents, Segmentation, Competitive Landscape, Report Structure, Methodology.
Request a sample copyof this report: https://www.coherentmarketinsights.com/insight/request-sample/1960
Orphan drug designation grantedby regulatory agencies such as the Food and Drug Administration (FDA) andEuropean Medicine Agency (EMA) for the treatment of PSC enables manufacturersto accelerate the drug development process for providing new drug treatments topatients in need. For instance, in 2017, Conatus Pharmaceuticals Inc. wasgranted orphan designation to IDN-7314 by the FDA and EMA. The drug completedtwo successful pre-clinical trials, of which the second one was completed in2017. Also, in 2016, the U.S. Food and Drug Administration (FDA) granted OrphanDrug Designation for BTT1023 drug candidate developed by Acorda Therapeutics,which is in Phase 2 clinical trials currently.
Also, pharmaceuticals companiesare focusing on mergers and acquisitions to accelerate the development of newtreatment options to address the unmet needs of patients suffering from rarediseases by advancing the therapies into clinical trials. For instance, in2015, Gilead Sciences, Inc. acquired Phenex Pharmaceuticals AG, to gain accessto Phenex’s Farnesoid X Receptor (FXR) program comprising small molecule FXRagonists for the treatment of liver diseases including PSC and nonalcoholicsteatohepatitis (NASH). Also, in February 2018, Intercept Pharmaceuticals, Inc.collaborated with Sumitomo Dainippon Pharma Co. Ltd., to develop andcommercialize Obeticholic Acid (OCA) for chronic liver disease in Japan, China,and Korea.
In 2014, Shire acquired LumenaPharmaceuticals, to gain access to its late-stage rare disease pipeline assetsindicated for gastrointestinal conditions- LUM001 and LUM002, thus providingpotential treatment options to patients with rare cholestatic liver disease andadding products to its rare disease portfolio.
Browse ResearchReport: https://www.coherentmarketinsights.com/market-insight/primary-sclerosing-cholangitis-market-1960
Key Takeaways of the PrimarySclerosing Cholangitis Market:
According to the ResearchInstitute of Radiological Science, 2016, the prevalence of PSC is higher inNorthern Europe and the U.S. around 10 per 100,000 population, while it is farless common in Southern Europe and Asia
Requirement of providingaffordable treatment, owing to high costs associated with liver transplantationcoupled with increasing risk factors resulting in the development of seriousdisorders are factors that are expected to bolster market growth in the nearfuture.
Some of the major playersoperating in the global primary sclerosing cholangitis market include AcordaTherapeutics, Inc., Gilead Sciences, Inc., NGM Biopharmaceuticals, Inc.,Intercept Pharmaceuticals, Inc., Dr. Falk Pharma GmbH, Allergan Plc., ShirePlc., Durect Corporation, Conatus Pharmaceuticals, Inc., Sirnaomics, Inc., andShenzhen HighTide Biopharmaceutical Ltd.
Buy-Now this researchreport: https://www.coherentmarketinsights.com/insight/buy-now/1960
AboutCoherent Market Insights:
CoherentMarket Insights is a prominent market research and consulting firm offeringaction-ready syndicated research reports, custom market analysis, consultingservices, and competitive analysis through various recommendations related toemerging market trends, technologies, and potential absolute dollaropportunity.
ContactUs:
mailto:sales@coherentmarketinsights.com
U.S.Office:
Name: Mr. Shah
CoherentMarket Insights 1001 4th Ave,
# 3200Seattle, WA 98154, U.S.
US : +1-206-701-6702
UK : +44-020-8133-4027
JAPAN: +050-5539-1737