views
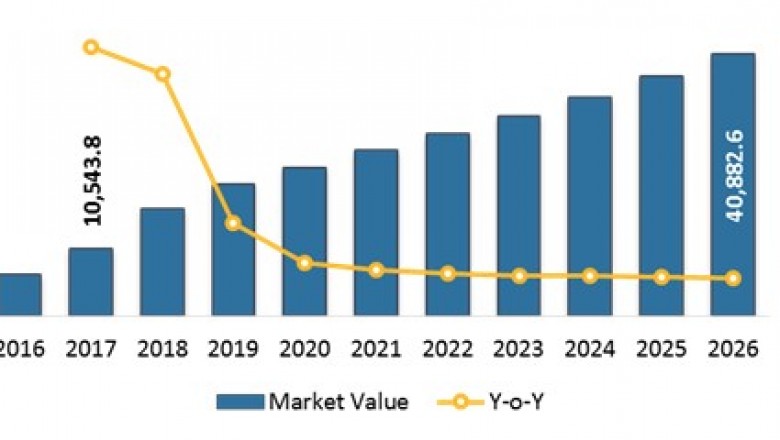
The global ImmuneCheckpoint Inhibitors Market by Drug Class (Programmed Death Receptor-1(PD-1) Inhibitors (Pembrolizumab (Keytruda), Nivolumab (Opdivo), Cemiplimab(Libtayo), Others (Spartalizumab), Programmed Death-Ligand 1 (PD-L1) Inhibitors(Atezolizumab (Tecentriq), Avelumab (Bavencio), Durvalumab (Imfinzi)),Cytotoxic T-Lymphocyte Antigen 4 (CTLA-4) Inhibitors (Ipilimumab (Yervoy)),Indoleamine-2,3-dioxygenase (IDO) Inhibitors, Lymphocyte-Activation Gene 3Inhibitors)), By Cancer Type (Lung Cancer, Head & Neck Cancers, Skin Cancer(Melanoma and Merkel Cell Carcinoma), Blood Cancer (Lymphoma), Bladder Cancer(Urothelial Carcinoma), Renal/Kidney Cancer, Colorectal Cancer, Breast Cancer,and Others), by Distribution Channel (Hospital Pharmacies, Retail Pharmacies,and Online Pharmacies), and Region (North America, Latin America, Europe, AsiaPacific, the Middle East, and Africa) was valued at US$ 10,543.8 million in2017, and is projected to exhibit a CAGR of 11.8% over the forecast period(2018 – 2026).
Increasing prevalence of canceris expected to boost the demand for immune checkpoint inhibitors. Also,innovative drug launches along with robust pipeline is expected to boost theglobal immune checkpoint inhibitors market growth over the forecast period.Major manufacturers are investing into R&D to develop immune-checkpointtherapies by understanding tumor functions and ways to combat them.Manufacturers are focusing on upgrading available immune checkpoint inhibitorsas well as developing new immune checkpoint inhibitors for cancer treatment.For instance, AstraZeneca’s Durvalumab (Imfinzi) was approved in 2017, asimmune checkpoint inhibitor, which blocks interaction of PD-L1 with PD-1 andCD80. In December 2017, Bristol-Myers Squibb received approval for Nivolumab(Opdivo) in adjuvant treatment of melanoma. In March 2017, Avelumab (Bavencio),jointly developed by EMD Serono, and Pfizer, Inc. received U.S. Food & DrugAdministration (FDA) approval for the treatment of metastatic merkel cellcarcinoma. In 2017, the U.S. FDA granted accelerated approval to immunotherapyproduct- TECENTRIQ (atezolizumab) for the treatment of patients with locallyadvanced or metastatic urothelial carcinoma (mUC).
* The sample copy includes: Report Summary, Table ofContents, Segmentation, Competitive Landscape, Report Structure, Methodology.
Request a sample copyof this report: https://www.coherentmarketinsights.com/insight/request-sample/2560
Browse 46 Market Data Tables and41 Figures spread through 238 Pages and in-depth TOC on Global ImmuneCheckpoint Inhibitors Market by Drug Class (Programmed Death Receptor-1 (PD-1)Inhibitors (Pembrolizumab (Keytruda), Nivolumab (Opdivo), Cemiplimab (Libtayo),Others (Spartalizumab), Programmed Death-Ligand 1 (PD-L1) Inhibitors(Atezolizumab (Tecentriq), Avelumab (Bavencio), Durvalumab (Imfinzi)),Cytotoxic T-Lymphocyte Antigen 4 (CTLA-4) Inhibitors (Ipilimumab (Yervoy)),Indoleamine-2,3-dioxygenase (IDO) Inhibitors, Lymphocyte-Activation Gene 3Inhibitors)), By Cancer Type (Lung Cancer, Head & Neck Cancers, Skin Cancer(Melanoma and Merkel Cell Carcinoma), Blood Cancer (Lymphoma), Bladder Cancer(Urothelial Carcinoma), Renal/Kidney Cancer, Colorectal Cancer, Breast Cancer,and Others), by Distribution Channel (Hospital Pharmacies, Retail Pharmacies,and Online Pharmacies), and region(North America, Latin America, Europe, Asia Pacific, the Middle East, andAfrica) - Global Forecast to 2025
Research and development byleading as well as small and mid-sized players in immune checkpoint inhibitorsmarket is expected to support global immune checkpoint inhibitors marketgrowth. For instance, Oncolytics Biotech, Inc. announced research collaborationwith the Keck School of Medicine of University of Southern California (USC), inorder to develop a combination therapy of Reolycin (Oncolytics Biotech’sproduct), Keytruda, Velcade, and dexamethasone for the treatment of multiplemyeloma in May 2018. Furthermore, in January 2018, Keytruda, developed by Merck& Co. reported slow liver cancer progression in Phase 2 trial.Immuno-oncology combination therapies are also under research for variouscancer indications.
Browse ResearchReport: https://www.coherentmarketinsights.com/market-insight/immune-checkpoint-inhibitors-market-2560
In January 2018, the U.S. Food& Drug Administration (FDA) granted breakthrough therapy status toLenvima-Keytruda combo for advanced kidney cancer. Furthermore, in February2018, Opdivo-Yervoy combination therapy showed delayed disease progression inpatients with advanced non-small cell lung cancer. Moreover, Genentechcombination therapy Tecentriq and Avastin delayed kidney cancer progression inPhase III trials in December 2017. AstraZeneca Plc’s Imfinzi (Durvalumab)showed delayed non-small cell lung cancer progression in Phase III trials inNovember 2017.
Key Takeaways of the Global ImmuneCheckpoint Inhibitors Market:
The global immune checkpointinhibitors market is expected to exhibit a CAGR of 11.8% over the forecastperiod. This is attributed to presence of several leading manufacturers who arefocusing on introducing innovative therapies through extensive research anddevelopment such as Bristol Myers Squibb, Novartis, and Pfizer, Inc.
Biopharmaceutical companies aredeveloping a robust pipeline of immune-checkpoint inhibitor combinationtherapies due to their increasing demand. The U.S. Food & DrugAdministration (FDA) has approved a number of immune checkpoint inhibitorsincluding Yervoy (anti-CTLA-4), Opdivo and Keytruda (anti-PD1) and Tecentriq(anti-PD-L1).
Immuno-checkpoint inhibitorcombination therapies are expected to change the market scenario over theforecast period, owing to positive results in the clinical trials
Research partnerships and collaborationsto develop new drugs by various market players is supporting growth of themarket, as competitors are striving to gain competitive edge in the market
Major players operating in theglobal immune checkpoint inhibitors market include Bristol-Myers SquibbCompany, Merck & Co., Inc., F. Hoffmann-La Roche AG, AstraZeneca Plc.,Novartis International AG, ImmunOs Therapeutics AG, Immutep Ltd., NewLinkGenetics Corporation, Ono Pharmaceutical Co., Ltd., and Pfizer, Inc.
Buy-Now this researchreport: https://www.coherentmarketinsights.com/insight/buy-now/2560
AboutCoherent Market Insights:
CoherentMarket Insights is a prominent market research and consulting firm offeringaction-ready syndicated research reports, custom market analysis, consultingservices, and competitive analysis through various recommendations related toemerging market trends, technologies, and potential absolute dollaropportunity.
ContactUs:
mailto:sales@coherentmarketinsights.com
U.S.Office:
Name: Mr. Shah
CoherentMarket Insights 1001 4th Ave,
# 3200Seattle, WA 98154, U.S.
US : +1-206-701-6702
UK : +44-020-8133-4027
JAPAN : +050-5539-1737