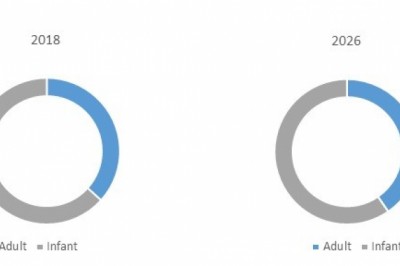
views
ElectricalStimulation Blood Pressure Treatment Devices Market - Global IndustryInsights, Trends, Outlook, and Opportunity Analysis, 2018-2026
Electrical stimulation technologyhas been in use for various applications such as pain management, neurologicaland movement disorder management, musculoskeletal disorder management, andmetabolism & GIT management. However, products for the treatment ofresistance hypertension are under development. Despite significant advances inthe drug therapy, hypertension still remains a major clinical condition and akey factor in the cardiovascular disease. Even changes in lifestyle habits suchas weight loss, exercise, reduced alcohol consumption, and dietary sodiumrestriction are not sufficient. Thus new strategies and algorithms for the treatmentof hypertension are required.
Various alternative treatmenttherapies are available such as renal denervation, the ROX coupler, andbaroreflex activation therapy. Renal denervation involves the catheter-basedablation of both afferent nerves and efferent nerves. The technology lookedpromising until 2014 when one of the major trials failed to meet the primaryend points. However, companies like Medtronic and Boston Scientific areconducting new trials using modified renal denervation catheters. The ROXcoupler on the other hand is surgically implanted between a vein and artery inthe upper thigh, in a procedure called arteriovenous anastomosis.
* The sample copy includes: Report Summary, Table ofContents, Segmentation, Competitive Landscape, Report Structure, Methodology.
Request a sample copyof this report: https://www.coherentmarketinsights.com/insight/request-sample/25
Baroreflex Activation Therapy
The baroreflex activation therapyis a modified version of the earlier Rheos Baroreflex Activation Therapysystem. The concept behind electrical stimulation of baroreceptors orbaroreflex afferent nerves is that the stimulus is sensed by the brain as theblood pressure increases. Then, baroreflex efferent structures are adjusted tocounteract the perceived blood pressure increase. The Barostim neo system fromCVRx is the most touted device in this category. The device has a CE Mark,while the U.S. FDA has granted the technology an Expedited Access Pathwaydesignation. The device is under investigation for application in heartfailure.
This new electrical stimulationimplant could be a good alternative treatment option to drugs in blood pressurepatients. The fear of side effect can be overcome by using this implantabledevice. Moreover, this device offers benefits such as non-destructive andreversible treatment, 100% treatment compliance and meet every patient needs.
Market Scenario
Prevalence of hypertension ishighest among the low and middle income countries in Africa, Latin America, andSouth-East Asian regions according to the World Health Organization (WHO).Moreover, inadequate market penetration, large patient pool and aging populationare the major factors that will drive the demand for electrical stimulationdevices for the treatment of blood pressure in these regions. Moreover,hypertension is also one of the major causes of morbidity and mortality in theUnites States. In Canada, almost 20% of the hypertension patients have theirblood pressure above the normal level in spite of using an optimal combinationof at least three antihypertensive drugs. This statistics by the CanadianAgency for Drugs and Technologies in Health (CADTH) is of the opinion thatcollaboration between clinical specialties will be needed to appropriatelyidentify patients who may benefit from this procedure.
Browse ResearchReport: https://www.coherentmarketinsights.com/ongoing-insight/electrical-stimulation-blood-pressure-treatment-devices-market-25
The electrical stimulationdevices for blood pressure are under trials to establish their effectiveness.Barostim neo system has received humanitarian device exemption for use inpatients with resistant hypertension from the U.S. FDA. However, it can only beused in patients who had received implantation of the earlier generation,bilateral Rheos Carotid Sinus Leads (now discontinued) during clinical trials.Successful outcomes from the current trials will expedite the commercializationof the device in next five years.
Key developments
Key players are involved invarious business strategies such as participating in international conferencesand medical fairs to market their products. For instance, in April 2018, BTLIndustries, Inc. participated at 79th China International Medical EquipmentFair (CIMEF) in Shanghai, which was the largest fair of medical equipment andservices in Asia Pacific and second largest in the world.
Key players in the market areinvolved in gaining approvals for devices from regulatory authorities, in orderto provide efficient devices in the market. For instance, in 2018, Medtronicplc. announced the U.S. Food and Drugs Administration (FDA) approval to beingan investigational device exemption (IDE) pivotal trial to evaluate theSymplicity Spyral (TM) renal denervation system in patients with high bloodpressure i.e. hypertension.
Buy-Now this researchreport: https://www.coherentmarketinsights.com/insight/buy-now/25
AboutCoherent Market Insights:
CoherentMarket Insights is a prominent market research and consulting firm offeringaction-ready syndicated research reports, custom market analysis, consultingservices, and competitive analysis through various recommendations related toemerging market trends, technologies, and potential absolute dollaropportunity.
ContactUs:
mailto:sales@coherentmarketinsights.com
U.S.Office:
Name: Mr. Shah
CoherentMarket Insights 1001 4th Ave,
# 3200Seattle, WA 98154, U.S.
US : +1-206-701-6702
UK : +44-020-8133-4027
JAPAN : +050-5539-1737