views
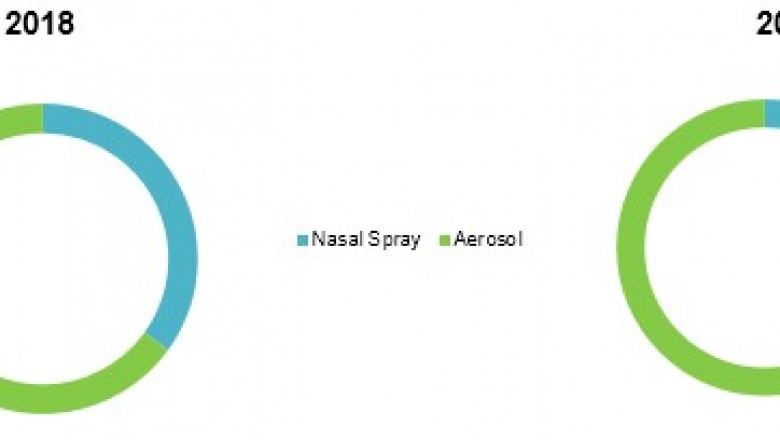
The Global CiclesonideMarket, by Indication (Asthma and Allergic Rhinitis), by Dosage Form(Aerosol and Nasal Spray), and by Region (North America, Latin America, Europe,Asia Pacific, Middle East, and Africa) was valued at US$ 565.4 million in 2018,and is projected to exhibit a CAGR of 1.9% over the forecast period (2018 –2026).
Inhaled corticosteroids (ICS)such as ciclesonide are at the cornerstone of treatment in asthma and inchronic obstructive pulmonary disease (COPD). The majority of the asthmapatients, are treated with a combination of ICS and short- or long- actingbronchodilators. High efficiency of inhaled corticosteroids in treating asthmaand COPD incidences is supporting adoption of ciclesonide in the future. Forinstance, according to the study findings published in the BMJ Journal, in November2018, use of triple therapy consisting of a long acting muscarinic antagonist(LAMA), long acting β agonist (LABA), and inhaled corticosteroid (ICS);resulted in lower rate of moderate or severe exacerbations of COPD, better lungfunction, and better health related quality of life than dual therapy ormonotherapy in patients with advanced COPD.
Browse 26 Market Data Tables and24 Figures spread through 126 Pages and in-depth TOC on ‘Ciclesonide Market, byIndication (Asthma and Allergic Rhinitis), by Dosage Form (Aerosol and NasalSpray), and by Region (North America, Latin America, Europe, Asia Pacific,Middle East, and Africa) - Global Forecast to 2026’
* The sample copy includes:Report Summary, Table of Contents, Segmentation, Competitive Landscape, ReportStructure, Methodology.
Request a sample copyof this report: https://www.coherentmarketinsights.com/insight/request-sample/2291
Launches and approvals of newciclesonide products in key regions is expected to create a conduciveenvironment for growth of the ciclesonide market over the forecast period. Forinstance, in 2012, Sunovion Pharmaceuticals Inc. received the U.S. Food andDrug Administration approval for its Zetonna (ciclesonide) nasal aerosol forthe treatment of allergic rhinitis and launched it in the U.S. market. In 2012,Nycomed, a subsidiary of Takeda Pharmaceutical Company Ltd., launched Omnaris(ciclesonide) nasal spray, indicated for the treatment of adults andadolescents over the age of 12 with allergic rhinitis in the United ArabEmirates (UAE). Key players in the market are involved in strategic mergers andacquisition strategies to enter the lucrative market of ciclesonide and towiden its portfolio with various ciclesonide products. Such strategic mergersand acquisitions are expected to support growth of ciclesonide market over theforecast period. For instance, in 2015, a U.K. based AstraZeneca Plc. acquiredTakeda Pharmaceutical Company Ltd.’s lung business and widen its offerings withAlvesco, Omnaris ciclesonide. In 2017, Sunovion Pharmaceuticals Inc. (Sunovion)divested its U.S. market rights of Sunovion’s ciclesonide products: ALVESCO(ciclesonide) Inhalation Aerosol, OMNARIS (ciclesonide) Nasal Spray, andZETONNA (ciclesonide) Nasal Aerosol to Covis Pharma B.V. (Covis Pharma).
Browse ResearchReport: https://www.coherentmarketinsights.com/market-insight/ciclesonide-market-2291
Key Takeaways of the CiclesonideMarket:
The global ciclesonide market isexpected to exhibit a CAGR of 9% over the forecast period, owing to increasingengagement of key players to gain higher market share by employing organic andin-organic strategies such as new product launch and acquisition and divestment.
Among indication, allergicrhinitis segment accounted for major market share in 2017. According to thedata published by Allergy UK, allergic rhinitis is a very common non-infectiousrhinitis and in the U.K. it affects 10% and 30% of all adults and as many as40% of children in 2013. As per the same source, around 57% of adult patientsand up to 88% of children with allergy rhinitis have sleep problems, includingmicro-arousals. Such high prevalence of allergic rhinitis is expected todrastically increase demand for ciclesonide, which is widely used in thetreatment of allergy rhinitis.
Among dosage form, aerosolsegment accounted for highest revenue in 2017, in the market, owing to easy anddirect inhalation of ciclesonide through aerosol, allowing to deliverappropriate quantity medication to the patient. Furthermore, aerosol therapies presentin the market are widely used for treatment of breathing conditions, includingasthma in adult and adolescent patients aged 12 years and above and symptomsassociated with perennial allergic rhinitis (PAR) and seasonal allergicrhinitis (SAR) in adults and adolescents 12 years of age and older.
Some of the major playersoperating in the global ciclesonide market include AstraZeneca Plc, CiplaLimited, Sun Pharmaceutical Industries Ltd., and Apotex Inc.
Buy-Now this researchreport: https://www.coherentmarketinsights.com/insight/buy-now/2291
AboutCoherent Market Insights:
CoherentMarket Insights is a prominent market research and consulting firm offeringaction-ready syndicated research reports, custom market analysis, consultingservices, and competitive analysis through various recommendations related toemerging market trends, technologies, and potential absolute dollaropportunity.
ContactUs:
mailto:sales@coherentmarketinsights.com
U.S.Office:
Name: Mr. Shah
CoherentMarket Insights 1001 4th Ave,
# 3200Seattle, WA 98154, U.S.
US : +1-206-701-6702
UK : +44-020-8133-4027
JAPAN : +050-5539-1737