views
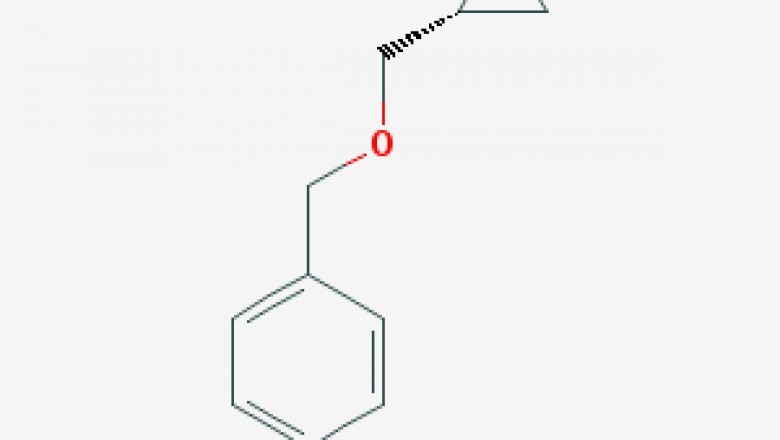
Molecular Formula ?C10H12O2
Molecular Mass ?164.2
Exact Mass ?164.083725
PSA ?21.8 A^2
LogP ?1.3
InChIKeys ?QNYBOILAKBSWFG-JTQLQIEISA-N
H-bond Acceptor ?2
H-bond Donor ?0
SP3 ?0.40
RBN ?4
Chemical Products (R)-Benzyloxymethyl-oxirane Basic Attributes
CAS No: 14618-80-5
Formula: C10H12O2
China Export: From 2018.11 to 2019.11, total export volume of (R)-Benzyloxymethyl-oxirane from China was 13,136,469KG while total export value was $93,749,048. The biggest proportion of exporting volume in the last 12 months was 12.79% in 2019.03.
Chemical Products (R)-Benzyloxymethyl-oxirane Basic Attributes
CAS No:14618-80-5
Molecular Formula :C10H12O2
Molecular Mass :164.2
Exact Mass :164.083725
PSA :21.8 A^2
LogP :1.3
InChIKeys :QNYBOILAKBSWFG-JTQLQIEISA-N
H-bond Acceptor :2
H-bond Donor :0
SP3 :0.40
RBN :4
Chemical Products (R)-Benzyloxymethyl-oxirane Characteristics
Density :1.077
Bolling Point :130 °C (0.1 mmHg)
Flash Point :>230 °F
Refractive Index :1.517
Solubility :soluble in water.
Storage Condition :2-8°C
BRN :3588399
Specific Rotation :-5.4 º (c=5 in toluene)
Chemical Products (R)-Benzyloxymethyl-oxirane Safety Information
HS Code :2910900090
UN No. :NONH for all modes of transport
WGK_Germany :3
Risk Code :36/37/38
Safety Instructions :26-36-37/39
RTECS No. :TX2860020
Dangerous Mark :Xi
P Code :P261-P305 + P351 + P338
Hazard Statements :H315-H319-H335
Chemical Products (R)-Benzyloxymethyl-oxirane Product Usage
Used in the preparation of lactone fragments of compadine and lovastatin. 1 Preparation of cis-1,3-polyol, 2 dideoxy nucleoside 3 and spiroacetal cyanohydrin chiral.
Chemical Products (R)-Benzyloxymethyl-oxirane Production Methods
Under an argon atmosphere, to a suspension of NaH (179 mg, 4.10 mmol, 55percent in oil) in THF (2 mL) were sequencially added a solution of (S)-glycidol (S1, 200 mg, 2.70 mmol) in THF (4 mL), and benzyl bromide (0.340 mL, 2.86 mmol) at 0 °C. After the mixture was stirred for 19 h under reflux, the reaction was quenched with 0.1 M phosphate buffer (pH 6.5). The products were extracted with AcOEt (x3), and the combined organic layer was washed with brine, then dried over Na(R, R)-N, N-Bis-(3, 5-di-tert-butylsalicyclidene)-1, 2-cyclohexanesanediaminocobalt (II) (0.029 g, 0.05 mmol) was added to a mixture of 2-((benzyloxy)methyl)oxirane (1.63 g, 9.91 mmol), AcOH (0.011 mL, 0.198 mmol) and THF (0.15mL). The mixture was cooled to 0 °C and treated with H0.5 mole of racemic 1, 2-epoxy compounds were added with 0.4 mole percent of the catalyst prepared in Preparation [(1-RR)-(Dibenzoyl-LTA) ] (or Preparation [ (1-SS) - (Dibenzoyl- DTA)]) and cooled down to 5 To a stirred solution of(S)-glycidol (5 g, 67.5 mmol) and benzyl chloride (13 g) in DMF (40 mL) cooled at 0 °C was added a powder of NaH (2.4 g) slowly for 10 minutes.
Chemical Products (R)-Benzyloxymethyl-oxirane Material and Products
Material :
N,N-Dimethylformamide, (S)-Oxiranemethanol, BENZYLGLYCIDYLETHER, ALLYL BENZYL ETHER, Petroleum Ether, Ethyl Acetate, Benzylbromide, Sodium Hydride