views
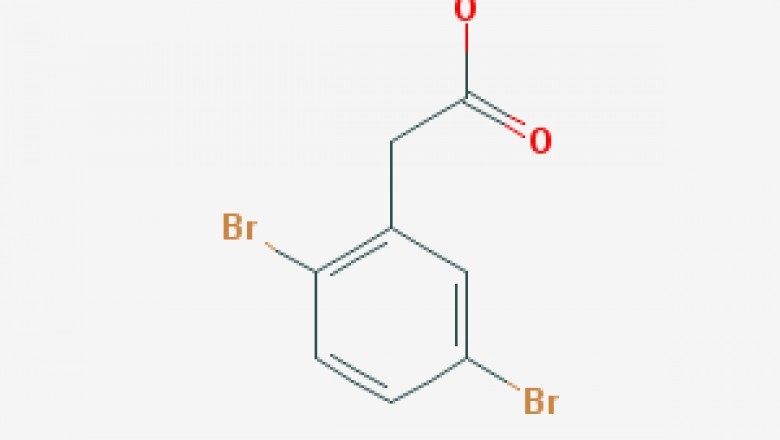
Molecular Formula ?C8H6Br2O2
Molecular Mass ?293.94004
Exact Mass ?291.873444
PSA ?37.3 A^2
LogP ?2.8
EINECS ?804-900-1
InChIKeys ?NKZKFWYEXDBOTP-UHFFFAOYSA-N
H-bond Acceptor ?2
H-bond Donor ?1
SP3 ?0.13
RBN ?2
Chemical Products 2,5-Dibromophenylacetic acid Basic Attributes
CAS No:203314-28-7
Formula:C8H6Br2O2
Synonyms:2,5-Dibromophenylacetic acid;2-(2,5-DibroMophenyl)acetic acid;2,5-Dibromobenzeneacetic acid;Dibromophenylacetic acid
China Export:From 2018.11 to 2019.11, total export volume of 2,5-Dibromophenylacetic acid from China was 50,754,743KG while total export value was $481,956,961. The biggest proportion of exporting volume in the last 12 months was 11.87% in 2019.05.
Chemical Products 2,5-Dibromophenylacetic acid Basic Attributes
CAS No:203314-28-7
Molecular Formula :C8H6Br2O2
Molecular Mass :293.94004
Exact Mass :291.873444
PSA :37.3 A^2
LogP :2.8
EINECS :804-900-1
InChIKeys :NKZKFWYEXDBOTP-UHFFFAOYSA-N
H-bond Acceptor :2
H-bond Donor :1
SP3 :0.13
RBN :2
Chemical Products 2,5-Dibromophenylacetic acid Characteristics
Density :1.969±0.06 g/cm3
Flash Point :181.5±23.7 °C
Refractive Index :1.625
Solubility :H2O: Very slightly soluble (0.35 g/L) (25 ºC)
Chemical Products 2,5-Dibromophenylacetic acid Safety Information
HS Code :2916399090
P Code :P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P312, P304+P340, P305+P351+P338, P312, P321, P330, P332+P313, P337+P313, P362, P403+P233, P405, P501
Hazard Statements :H302+H332
Chemical Products 2,5-Dibromophenylacetic acid Production Methods
In a 500 ml four-neck reaction flask, 82 g of Intermediate 3 was added, and 400 ml of a 2N aqueous solution of sodium hydroxide was added and the mixture was heated to 100° C. and refluxed.TLC monitored the reaction, cooled to room temperature, added dropwise with 36percent hydrochloric acid to pH=8, and the aqueous layer was extracted with ethyl acetate.The aqueous layer was adjusted to pH=1 to 2 with 36percent hydrochloric acid, solids were precipitated, and filtered to obtain an off-white solid 2, 5-dibromophenylacetic acid, dried 73 g, yield 83percent.The 5-bromofluorenone 670.7 g obtained in the previous step, Add 1320ml of water and cool to -5-0 °C in an ice bath.Then add 1100g of hydrogen bromide (48percent) aqueous solution and stir.Most of the raw materials are dissolved, and the temperature is controlled within the range of -5-0 °C.Add 143 g of sodium nitrite to a solution of 286 ml of water.During the period, the control temperature does not exceed 0 °C, about 15 minutes, The incubation reaction was continued for 1 h, and a large amount of brown granular solid (diazonium salt) was precipitated in the reaction solution for use.In another 10000ml four-necked flask, 297g of cuprous bromide, 22g of copper bromide, 13200ml of water, hydrogen bromide(48percent) 1100g of aqueous solution, stirred, the reaction solution was dark red, heated to 20-25 ° C, the diazonium salt obtained in the previous step was added dropwise.After the completion of the dropwise addition, the reaction was continued for 1 h, the temperature was lowered to about 5 ° C, the temperature was kept for 0.5 h, and the filter cake was washed with cold water until the washing liquid was colorless. drying, The solid 2, 5-dibromophenylacetic acid 746 g was obtained in a yield of 80.5percent, and the HPLC purity was 98.8percent.300.0 g of bromoindolinone (Formula II) prepared in the previous step was put into a reaction flask, 600 ml of water was added, and the temperature was lowered to -5 to 0 ° C in an ice bath, Then 493.5g of hydrogen bromide (48percent) aqueous solution was added and stirred.