views

The global In vitro diagnostics market size is expected to reach USD 113.38 billion by 2030, according to a new report by Grand View Research, Inc. It is estimated to register a CAGR of 0.2% over the forecast period driven by the increasing geriatric population, COVID-19 pandemic, and technological advancements in diagnostics that are supporting its adoption. Technological advancements in terms of portability, accuracy, and cost-effectiveness are projected to be one of the high-impact rendering drivers. Technological advancements were further accelerated by the launch of COVID-19 IVD diagnostics and enhanced the adoption of instruments and consumables for technologies, such as PCR. Competitors in the market are increasingly adopting agreement and partnership strategies to maintain a constant flow of business for manufacturers & diagnostics for users.
These agreements are also a result of the harsh price containment strategies for government laboratories, which lowers the price in government settings. For instance, in April 2021, the Italian subsidiary of Seegene, Inc. received a USD 108.25 million tenders for public procurement for the supply of extraction reagents, as well as 7.15 million SARS-CoV-2 diagnostic tests. However, it increases the multiparty nature and complexity of the supply chain. The high prevalence of cancer and Cardiovascular Diseases (CVDs) globally is anticipated to drive diagnostic innovation to facilitate early diagnosis and meet the constantly evolving needs of consumers. Novel technologies, such as plasmonic PCR, are anticipated to commercially enter the market during the forecast period, influencing the business of existing products adversely.
In Vitro Diagnostics Market Report Highlights
• Molecular diagnostics is anticipated to grow at the fastest CAGR from 2022 to 2030 owing to the rising adoption and usage rate
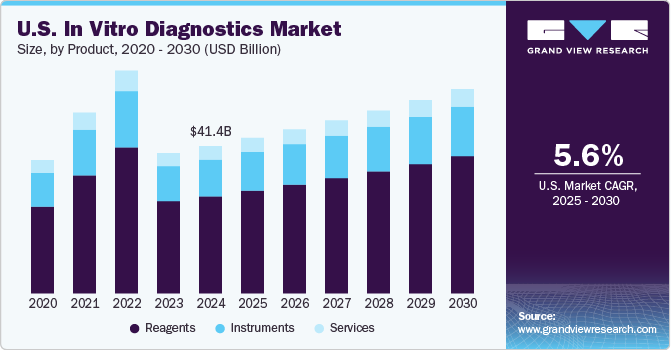
• Reagents held the largest market share owing to the surge in demand for genetic testing and enhanced availability of technologically advanced diagnostic tests in lower and middle-income countries with unmet clinical needs
• The infectious diseases application segment held the largest market share owing to the large volume of testing for infectious diseases globally
• North America dominated the global market in 2021 owing to the high demand for novel technologies, a large pool of key players, high prevalence of diseases, and advanced healthcare infrastructure
The global In Vitro Diagnostics (IVD) and IVD quality control markets combine to account for USD 112.79 billion in revenue in 2021, which is expected to reach USD 114.73 billion by 2030, growing at a cumulative rate of 0.2% over the forecast period.
In Vitro Diagnostics Market Segmentation
Grand View Research has segmented the global in vitro diagnostics market based on product, technology, application, end-use, test location, and region:
IVD Product Outlook (Number of Instruments Installed in Thousands, Number of Reagents Sold in Millions; Revenue, USD Million, 2018 - 2030)
• Instruments
• Reagents
• Services
IVD Technology Outlook (Number of Instruments Installed in Thousands, Number of Reagents Sold in Millions; Revenue, USD Million, 2018 - 2030)
• Immunoassay
o Instruments
o Reagents
o Services
• Hematology
o Instruments
o Reagents
o Services
• Clinical Chemistry
o Instruments
o Reagents
o Services
• Molecular Diagnostics
o Instruments
o Reagents
o Services
• Coagulation
o Instruments
o Reagents
o Services
• Microbiology
o Instruments
o Reagents
o Services
• Others
o Instruments
o Reagents
o Services
IVD Application Outlook (Revenue, USD Million, 2018 - 2030)
• Infectious Disease
• Diabetes
• Oncology
• Cardiology
• Nephrology
• Autoimmune Disease
• Drug Testing
• Others
IVD End-use Outlook (Number of Instruments Installed in Thousands, Number of Reagents Sold in Millions; Revenue, USD Million, 2018 - 2030)
• Hospitals
• Laboratories
• Home Care
• Others
IVD Test Location Outlook (Revenue, USD Million, 2018 - 2030)
• Point of Care
• Home Care
• Others
IVD Regional Outlook (Number of Instruments Installed in Thousands, Number of Reagents Sold in Millions; Revenue, USD Million, 2018 - 2030)
- North America
- S.
- Canada
- Europe
- K.
- Germany
- Spain
- France
- Italy
- Russia
- Asia Pacific
- Japan
- China
- India
- South Korea
- Singapore
- Australia
- Latin America
- Brazil
- Mexico
- Argentina
- Middle East and Africa (MEA)
- South Africa
- UAE
- Saudi Arabia
Order a free sample of In Vitro Diagnostics And IVD Quality Control Industry Data Book @ https://www.grandviewresearch.com/sector-report/in-vitro-diagnostics-ivd-quality-control-industry-data-book/request/rs1
List of Key Players in In Vitro Diagnostics Market
• Abbott
• Bio-Rad Laboratories, Inc.
• Quest Diagnostics
• bioMérieux SA
• QIAGEN
• Sysmex Corp.
• Agilent Technologies












