views

The fetal monitoring market is expanding with the latest innovative technologies, backed by escalating research and product launches. Fetal monitoring technologies are now being streamlined to provide greater assistance in utero fetal surgeries, consisting of challenging physical fetal access and technological complications. Fetal cardiac assessments have also witnessed progress that supports high-fidelity hemodynamic and continuous physiologic monitoring, thus enabling early diagnosis and treatment.
Computer-Aided Decision Support Systems and Artificial Intelligence (AI) are also utilized in continuous cardiotocography (CTG) or fetal heart rate (FHR) auscultation during labor. The patient’s electronic health records (EHRs) are utilized along with computational methods, machine learning, and deep learning tools.
Research and technological advancements in fetal monitoring systems have increased focus on noninvasive monitoring procedures. Obstetrics has been introduced with noninvasive diagnosis technologies such as Doppler studies, cell-free fetal DNA assessment, electronic fetal monitoring (EFM), and fetal acid-base status. Fetal and neonatal assessments are carried out during antepartum via percutaneous umbilical cord blood sampling and during intrapartum with fetal scalp blood sampling immediately after birth. Fetal phonocardiography has been integrated with advanced data acquisition systems and databases.
Download PDF Brochure @ https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=35700261
The advanced technology integrated into fetal monitors comes at a heavy price. Sophisticated monitoring techniques such as Doppler ultrasound machines also incur hefty charges. The probes used in the Doppler technology (for example, the Philips Doppler transducer) range from USD 4,500 to USD 17,000. The SonoScape system from GE Healthcare costs around USD 9,000.
The key manufacturers in fetal monitoring market are expanding their product lines to include portable and wireless monitoring systems that are accurate, safe, and affordable. The key players in the market are also integrating telecommunication into these devices. For instance, Philips launched the handheld and tele-ultrasound solution, Lumify, in Japan and Avalon CL in the US in 2020.
Similarly, the strategic collaboration between MindChild Medical and Henry Schein Medical expanded the MERIDIAN M110 Fetal Monitoring System's distribution and supply, a noninvasive intrapartum monitor in the US, in 2019.
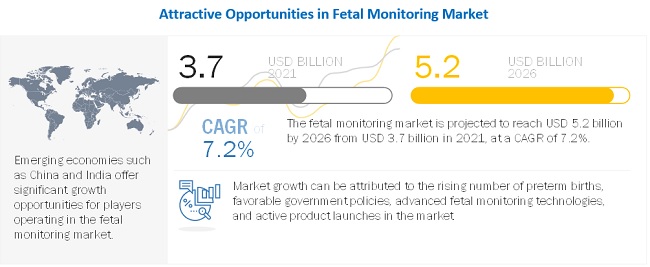
[228 Pages Report] The fetal monitoring market is projected to reach USD 5.2 billion by 2026 from USD 3.7 billion in 2021, at a CAGR of 7.2% from 2021 to 2026. The development of noninvasive, portable, and advanced fetal monitors and emerging markets and strengthening infrastructure are expected to offer growth opportunities in the market during the forecast period.












