views
Roots Analysis has done a detailed study on Cell Encapsulation: Focus on Therapeutics and Technologies, 2019-2030, covering key aspects of the industry’s evolution and identifying potential future growth opportunities.
To order this 500+ page report, which features 170+ figures and 395+ tables, please visit this - https://www.rootsanalysis.com/reports/view_document/cell-encapsulation-focus-on-therapeutics-and-technologies-2019-2030-/249.html
Key Market Insights
§ Presently, over 45 encapsulated cell therapies and cell encapsulation techniques are being evaluated across different phases of development by stakeholders across the world
§ Ongoing therapy development programs are evaluating different types of cells, encapsulated in a wide range of biocompatible materials, aiming to offer viable and effective treatment options for various diseases
§ In fact, majority of the product candidates are being developed for the treatment of metabolic disorders, primarily diabetes; big pharma are driving a significant proportion of research and development activity
§ Clinical research in this field is growing at a fast pace; encapsulated therapy products are evaluating a number of pre-marketing end points to validate safety / efficacy
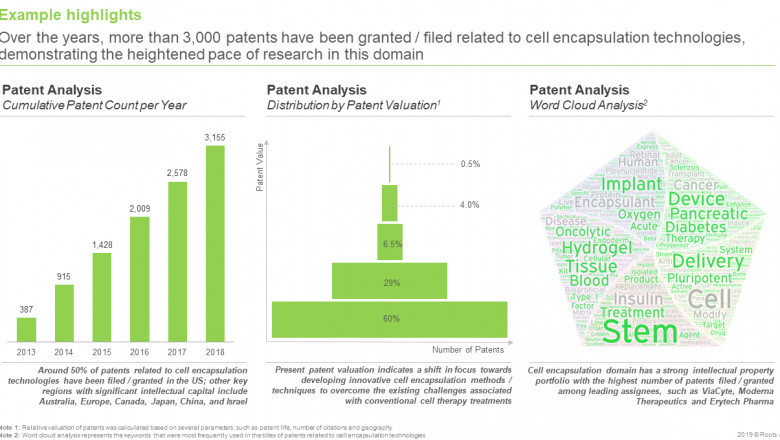
§ Over the years, more than 3,000 patents have been granted / filed related to cell encapsulation technologies, demonstrating the heightened pace of research in this domain
§ Foreseeing a lucrative future, several private and public investors have made capital investments worth approximately USD 1 billion, across over 100 funding instances, since 2013
§ Growth in partnership activity reflects the rising interest of stakeholders in this domain; over 70% of deals have been inked related to therapies for metabolic disorders, involving both international and indigenous parties
§ An evaluation of more than 300+ stakeholders engaged in cell therapies domain reveals the presence of several likely strategic partners spread across different geographical regions
§ The short term opportunity in this market is likely to be driven by licensing activity and will depend on the untapped potential of novel cell encapsulation technologies in different application areas
§ As multiple mid-late stage encapsulated cell therapies get commercialized in near future across different regions, the long term opportunity is likely to be distributed across diverse indications and encapsulation materials
§ The enormous potential of encapsulated cell-based therapies / devices in the treatment of chronic disorders has captured the interest of several stakeholders in the industry
For more information, please visit https://rootsanalysis.com/reports/view_document/cell-encapsulation-focus-on-therapeutics-and-technologies-2019-2030/249.html
Table of Contents
1. PREFACE
1.1. Scope of the Report
1.2. Research Methodology
1.3. Chapter Outlines
2. EXECUTIVE SUMMARY
3. INTRODUCTION
3.1. Context and Background
3.2. An Overview of Cell Therapies
3.2.1. Cell Therapy Manufacturing
3.2.2. Supply Chain
3.2.3. Key Challenges
3.3. An Introduction to Cell Encapsulation
3.3.1. Historical Overview
3.3.2. Cell Encapsulation Approaches
3.3.3. Encapsulation Materials
3.3.4. Advantages and Challenges
3.4. Potential Applications of Cell Encapsulation
3.4.1. Targeted Drug / Therapy Delivery
3.4.2. Immunoprotection
3.4.3. Storage and Transportation
3.5. Key Growth Drivers and Road-blocks
4. CURRENT MARKET LANDSCAPE
4.1. Chapter Overview
4.2. Encapsulated Cell Therapies and Encapsulation Technologies: Developer Landscape
4.2.1. Distribution by Year of Establishment
4.2.2. Distribution by Geographical Location
4.2.3. Distribution by Size of Developers
4.2.4. Distribution by Type of Offering
4.3. Encapsulated Cell Therapies and Encapsulation Technologies: Development Pipeline
4.3.1. Distribution by Target Therapeutic Area
4.3.2. Distribution by Phase of Development
4.3.3. Distribution by Type of Cells and Other Encapsulated Components
4.3.4. Distribution by Type of Encapsulation Material Used
4.3.5. Distribution by Route of Administration
4.3.6. Distribution by Application Areas
4.4. Encapsulated Cell Therapies and Encapsulation Technologies: Initiatives of Big Pharmaceutical Players
5. ENCAPSULATED CELL THERAPIES AND ENCAPSULATION TECHNOLOGIES FOR METABOLIC DISORDERS: COMPANY PROFILES
5.1. Chapter Overview
5.2. Developers with Clinical Candidates
5.2.1. Beta-O2 Technologies
5.2.1.1. Company Overview
5.2.1.2. Financial Information
5.2.1.3. Product Description: ꞵAir Bio-artificial Pancreas
5.2.1.4. Recent Developments and Future Outlook
5.2.2. Diatranz Otsuka
5.2.2.1. Company Overview
5.2.2.2. Financial Information
5.2.2.3. Product Description: DIABECELL®
5.2.2.4. Recent Developments and Future Outlook
5.2.3. Sernova
5.2.3.1. Company Overview
5.2.3.2. Financial Information
5.2.3.3. Product Description: Cell Pouch System™
5.2.3.4. Recent Developments and Future Outlook
5.2.4. ViaCyte
5.2.4.1. Company Overview
5.2.4.2. Financial Information
5.2.4.3. Product Description: PEC-Direct™ and PEC-Encap™
5.2.4.4. Recent Developments and Future Outlook
5.3. Developers with Preclinical Candidates
5.3.1. ALTuCELL
5.3.2. Beta-Cell
5.3.3. Betalin Therapeutics
5.3.4. CellProtect Biotechnology
5.3.5. Defymed
5.3.6. Encellin
5.3.7. Kadimastem
5.3.8. PharmaCyte Biotech
5.3.9. Semma Therapeutics
5.3.10. Sigilon Therapeutics
5.3.11. Seraxis
5.3.12. SymbioCellTech
6. ENCAPSULATED CELL THERAPIES AND ENCAPSULATION TECHNOLOGIES FOR NON-METABOLIC DISORDERS: COMPANY PROFILES
6.1. Chapter Overview
6.2. Developers with Clinical Candidates
6.2.1. Azellon Cell Therapeutics
6.2.1.1. Company Overview
6.2.1.2. Financial Information
6.2.1.3. Product Description: Cell Bandage
6.2.1.4. Recent Developments and Future Outlook
6.2.2. EryDel
6.2.2.1. Company Overview
6.2.2.2. Financial Information
6.2.2.3. Product Description: EryDex System
6.2.2.4. Recent Developments and Future Outlook
6.2.3. Erytech Pharma
6.2.3.1. Company Overview
6.2.3.2. Financial Information
6.2.3.3. Product Description: GRASPA®
6.2.3.4. Recent Developments and Future Outloo
6.2.4. Gloriana Therapeutics
6.2.4.1. Company Overview
6.2.4.2. Financial Information
6.2.4.3. Product Description: EC-NGF
6.2.4.4. Recent Developments and Future Outlook
6.2.5. Living Cell Technologies
6.2.5.1. Company Overview
6.2.5.2. Financial Information
6.2.5.3. Product Description: NTCELL®
6.2.5.4. Recent Developments and Future Outlook
6.2.6. MaxiVAX
6.2.6.1. Company Overview
6.2.6.2. Financial Information
6.2.6.3. Product Description: MVX-ONCO-1












