views
Cancer therapy, such as targeted immunotherapies, such as CAR-T cell therapies, has recently surpassed surgery, chemotherapy, and radiation. These are drugs that target cancer cells and help the patient's immune system fight the tumour. T-cells derived from a patient's blood are modified in the lab with the addition of a special protein receptor on the T-cells that attack cancer cells, known as CAR-T cell therapy. Chimeric Antigen Receptor (CAR) is a unique receptor that attaches to a specific protein on cancer cells in patients. Hundreds of millions of CAR-T cells are generated in the lab before being infused into patients. These cells bind to infected cancer cells and have the power to kill them, resulting in the cancer being cured. Immune checkpoint inhibitors, which can detect and stop cancer cells, can be used to battle cancer cells masking themselves from immune cells. The treatment of children recurrent acute lymphoblastic leukaemia, refractory non-Hodgkin lymphoma, and other cancers has been successful with this unique strategy of employing the body's immune cells to target cancer-causing cells.
Since the first historic CAR-T approvals in 2017, the following CAR T-cell therapies have been approved: Kymriah (tisagenlecleucel), Yescarta (axicabtagene ciloleucel), Tecartus (brexucabtagene autoleucel), Breyanzi (lisocabtagene maraleucel), and Abecma (lisocabtagene maraleucel) (idecabtagene vicleucel). Relma-cel (relmacabtagene autoleucel) was also approved by the NMPA in China, according to JW Therapeutics. This is China's first CAR-T product to be independently created and approved as a Category 1 biologics product, and the world's sixth CAR-T product.
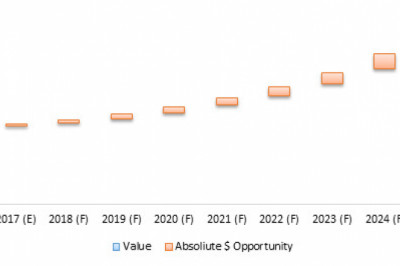
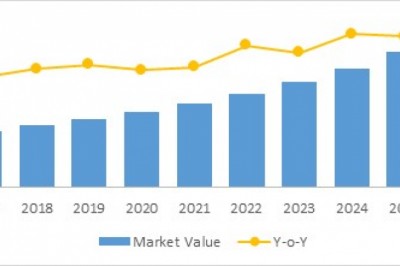
These ground-breaking approvals show that the CAR-T Cell Therapy market has arrived and is sweeping the biotech industry. CAR-T financing has soared to unprecedented heights as a result of this. Initially, the tendency was minor, but as CAR-T start-ups have been lavishly funded by investors eager to get into this fast expanding field of regenerative medicine, the tide has risen. In recent years, CAR-T firms have amassed a market valuation of over $100 billion.
Only 12 CAR-T clinical trials were underway in 2012. This figure had risen to more than 1,200 by the end of the day. The number of authorised CAR-T cell therapies is expected to reach double digits in the next five years, and more than 100 by 2035, based on CAR-T products in the clinical pipeline. The astounding 90% remission rate shown in acute lymphoblastic leukaemia (ALL) patients treated with Kymriah has spurred the increase in the number of clinical trials. Patients treated in the early clinical trials are now in remission for more than nine years in 60 percent to 70 percent of cases, which is an unparalleled achievement. Kymriah and Yescarta have been injected into almost half a million patients throughout the world since their first approvals.
Read More;
| https://bloggerstrive.blogspot.com/2021/12/car-t-cell-therapy-market-cancer.html |
https://www.coherentmarketinsights.com/market-insight/car-t-cell-therapy-market-102