views
With the emergence of blockbuster drugs, such as Gazyva® (for
Chronic Lymphocytic Leukemia) and POTELEGIO® (for Sézary syndrome), Fc
engineered antibodies have garnered significant interest in the medical
community, for various clinical conditions
London
Roots Analysis has announced the
addition of “Fc Protein and Glyco-engineered Antibodies Market,
2021-2030”
report
to its list of offerings.
Over time, a substantial body of evidence has validated the
therapeutic applications of Fc engineering platforms; Fc modified antibodies
have shown to augment the various immune effector functions, such as
antibody-dependent cellular cytotoxicity (ADCC), complement-dependent
cytotoxicity (CDC), antibody-dependent cellular phagocytosis (ADCP) activity
and / or the half-life of the molecule
To order this 250+ page report,
which features 100+ figures and 110+ tables, please visit https://www.rootsanalysis.com/reports/fc-protein-engineered-and-glycoengineered-antibodies-market.html
Key Market Insights
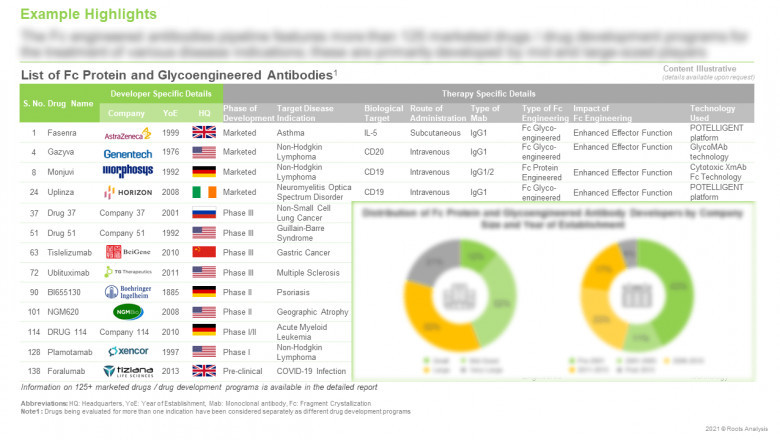
The Fc engineered antibodies pipeline features more
than 125 marketed drugs / drug development programs
Around 15% Fc protein and glyco-engineered antibodies are marketed,
while close to 75% are being evaluated in different phases of clinical trials
and 10% in preclinical studies. Examples of marketed Fc engineered antibodies include
Gazvya®, Imfinzi®, MONJUVI® and Skyrizi®.
Around
30 companies claim to be engaged in the development of Fc protein and glyco-engineered
antibodies
Around 55% of the aforementioned players are large companies
(with more than 5000 employees). It is worth highlighting that, majority of the
developers engaged in this domain (54%) are based in North America, followed by
Europe (26%) and Asia-Pacific (20%).
Over
1,800 clinical trials are currently evaluating the therapeutic effects of Fc
protein and glyco-engineered antibodies, worldwide
It is worth mentioning that most of the trials were / are being
conducted in North America (35%) region;
however, more than 91% of the patients enrolled in trials
conducted in North America were enrolled in different sites in the United
States. Further, 43% of the trials are being sponsored by non-industry players.
Close to 140 grants have been awarded to support research on Fc
protein and glyco-engineered antibodies, since 2019
An
estimated USD 63 million in grants have been awarded to various companies /
organizations working in this domain, during time period between 2019 and 2021.
Almost 50% of the total grant amount was funded by the National Institute of
Allergy and Infectious Diseases.
Close
to 6,500 patents have been filed / granted for Fc protein and glyco-engineered antibodies,
since 2016
Around 30%
of these intellectual property documents were filed / granted in the Asia-Pacific;
with maximum number of patents filed in Australia. This is followed by North
America (32%) and Europe (23%). Leading industry players (in terms of the size
of intellectual property portfolio) include Roche, Janssen, Novartis,
Genentech, Amgen, MacroGenics and Genmab.
Partnership
activity within this domain has grown at a CAGR of 48%, between 2016 and 2020
More than 50% of the total deals have been inked post 2019. Licensing
(36%) emerged as the most popular type of partnership model adopted by
stakeholders in this domain, followed by clinical trial agreements (16%) and acquisitions
/ mergers (14%).
The market is anticipated to grow at a CAGR of over 30%, during
the period 2021-2030
Growth in this domain is anticipated to be driven by the drugs
that are being developed for the treatment of oncological disorders. North
America (primarily the US) and Europe are expected to capture major share of
the Fc protein and glyco-engineered antibodies market by 2030, in terms of the
sales-based revenues.
To request a sample copy / brochure of
this report, please visit this https://www.rootsanalysis.com/reports/fc-protein-engineered-and-glycoengineered-antibodies-market/request-sample.html
Key Questions Answered
§ Who are the leading industry and non-industry players engaged in the development
of Fc protein and glyco-engineered antibodies?
§ Which are the key disease indications being targeted by Fc engineered
antibodies?
§ Which partnership models are commonly adopted by stakeholders engaged in
this domain?
§ Which geographies are the most active in conducting clinical trials on
Fc protein and glyco-engineered antibodies?
§ Which are the leading administering institutes supporting the research
related to Fc protein and glyco-engineered antibodies?
§ How has the intellectual property landscape in this market evolved over
the years?
§ Which key factors are likely to influence the evolution of this market?
§ How is the current and future market opportunity likely to be
distributed across key market segments?
The financial
opportunity within the Fc protein and glyco-engineered antibodies market has
been analyzed across the following segments:
§
Type of Fc
Engineering
§
Fc Protein
Engineering
§
Fc Glyco-engineering
§
Type of Therapy
§
Monotherapy
§
Combination Therapy
§
Therapeutic Area
§
Oncological Disorders
§
Rare Disorders
§
Dermatological Disorders
§
Autoimmune Disorders
§
Infectious Diseases
§
Gastrointestinal Disorders
§
Neurological Disorders
§
Pulmonary Disorders
§
Route of Administration
§
Intravenous
§
Subcutaneous
§
Others
§
Key Geographical Regions
§
North America
§
Europe
§
Asia-Pacific
§
Rest of the World
The research includes profiles of key players (listed below); each profile features an
overview of the company, its financial information (if available), brief
description of its drug(s), recent developments, and an informed future
outlook.
§
AbbVie
§
Alexion Pharmaceuticals
§
AstraZeneca
§
Genentech
§
MacroGenics
§
Kyowa Kirin
For additional details, please visit
or
email sales@rootsanalysis.com
You may also be interested in the following titles:
1.
TIL-based
Therapies Market,
2021-2030
2.
TCR-based
Therapies Market,
2021-2030
3.
Peptide
Therapeutics Market,
2021-2030
Contact:
Ben
Johnson
+1
(415) 800 3415












