views
To order this detailed 360+ page report, please visit https://www.rootsanalysis.com/reports/view_document/oligonucleotide-synthesis/304.html
Key Inclusions
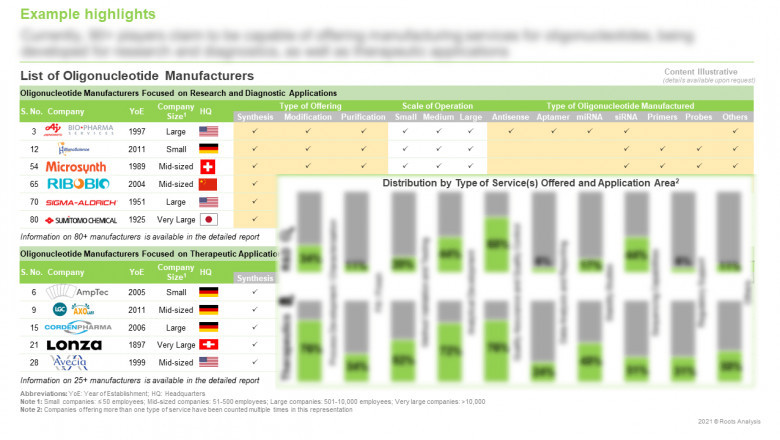
§ A detailed overview of the current market landscape of oligonucleotide manufacturers focused on research and diagnostic and therapeutic applications, based on several relevant parameters, such as year of establishment, company size, scale of operation (preclinical, clinical, and commercial), location of headquarters, number of manufacturing facilities, along with information on the location of facility (country-wise), regulatory accreditations and certifications received, type of oligonucleotide manufactured (antisense oligonucleotides, aptamers, decoys, miRNA, shRNA, siRNA, specialty amidites and others), type of offering (custom synthesis, modification and purification), type of manufacturing service(s) offered (process development and characterization, method validation and testing, analytical development, sequencing capabilities, stability studies, quality assurance and control, fill / finish, regulatory support, data analytics and reporting, and others), type of modification(s) offered (amino modifiers, backbone modifications, fluorescent probes, dyes and quenchers, modified bases, phosphorylation, thiol modifications and others) and type of purification method(s) used (desalting, cartridge purification, HPLC, ion exchange purification, PAGE and others).
§ An insightful company competitiveness analysis, highlighting the key oligonucleotide manufacturers focused on research and diagnostic, and therapeutic applications, based on supplier strength (on the basis of size of employee base and its experience) and service strength (on the basis of scale of operation, type of oligonucleotide manufactured, type of offering, type of manufacturing service(s) offered, type of modification(s) offered, type of purification method(s) used, and number and location of manufacturing facilities).
§ Elaborate profiles of key players engaged in offering custom synthesis, modification and purification of oligonucleotides for research and diagnostic, and therapeutic applications. Each profile includes an overview of the company, its financial performance (if available), information related to its service portfolio, manufacturing facilities, details on partnerships inked, recent developments and an informed future outlook.
§ A qualitative analysis highlighting the various factors that need to be considered by players engaged in this domain, while deciding whether to manufacture their respective products in-house or engage the services of a service provider.
§ A detailed review of the various oligonucleotide-based manufacturing initiatives undertaken by big pharma players (on the basis of revenues generated by top 5 pharmaceutical companies in 2020), highlighting trends across parameters, such as year of initiative, type of initiative and type of oligonucleotide.
§ An analysis of recent partnerships inked between players engaged in oligonucleotide manufacturing, during the period 2014-2021, based on several parameters, such as year of partnership, type of partnership, type of partner, most active players (in terms of number of partnerships signed), and region.
§ An analysis of the various expansion initiatives undertaken by service providers engaged in this domain, during the period 2016-2021, along with information on several relevant parameters, such as year of expansion, type of expansion (capability expansion, capacity expansion, facility expansion and new facility), scale of operation (preclinical, clinical and commercial), type of application (research and manufacturing operations), and location of manufacturing facility.
§ An in-depth analysis of over 90 oligonucleotide-based therapy developers that are likely to partner with contract service providers engaged in this domain, based on several relevant parameters, such as developer strength (on the basis of company size and its experience), pipeline strength and maturity (on the basis of number of drugs in pipeline and their stage of development) and other manufacturing capabilities.
§ A detailed analysis of completed and ongoing clinical research studies of various oligonucleotide-based drugs, based on several relevant parameters, such as trial registration year, phase of development, type of oligonucleotide, current trial recruitment status, study focus area, key therapeutic areas (in terms of number of trials undertaken / conducted), enrolled patient population and trial location, and leading industry and non-industry players (in terms of number of trials undertaken / conducted).
§ An estimate of the global, installed capacity for the manufacturing of oligonucleotides, based on information provided by various industry stakeholders in the public domain. It also features distribution of available oligonucleotide synthesis capacity on the basis of company size (small, mid-sized and large firms), scale of operation (preclinical, clinical and commercial), and key geographical regions (North America, Europe, Asia-Pacific and rest of the world).
§ An informed estimate of the annual commercial demand for oligonucleotide-based drugs (in kilograms), based on several relevant parameters, such as target patient population, dosing frequency and dose strength. The annual clinical demand for oligonucleotide-based drug products was also estimated, taking into account ongoing clinical trials.
§ An elaborate discussion on how the recent COVID-19 pandemic is likely to impact the oligonucleotide synthesis market, along with information on the key initiatives undertaken by players engaged in this domain to overcome the challenges faced due to the pandemic.
§ A discussion on affiliated trends, key drivers and challenges, under an elaborate SWOT framework, which are likely to impact the industry’s evolution, including a Harvey ball analysis, highlighting the relative effect of each SWOT parameter on the overall industry.
§ A survey analysis featuring inputs solicited from various experts who are directly / indirectly involved in providing custom synthesis, modification and purification services for oligonucleotides.
§ Informed estimates of the existing market size and the future opportunity for oligonucleotide synthesis, modification and purification services market, over the next decade. Based on multiple parameters, such as anticipated growth in number of oligonucleotide-focused projects, cost of goods sold, and direct manufacturing costs, we have developed informed estimates of the likely evolution of the market over the coming decade.
The report also features the likely distribution of the current and forecasted opportunity across important market segments, mentioned below:
- Application Area
§ Research and Diagnostic Applications
§ Therapeutic Applications
- Type of Oligonucleotide
§ Antisense Oligonucleotides
§ miRNA
§ shRNA
§ siRNA
§ Others
- Scale of Operation
§ Clinical
§ Commercial
- Purpose of Production
§ In-house Operations
§ Outsourced Operations
- Type of Operation
§ API
§ FDF
- Size of Manufacturer
§ Small
§ Mid-sized
§ Large
- Key Therapeutic Areas
§ Autoimmune Disorders
§ Cardiovascular Disorders
§ Genetic Disorders
§ Infectious Diseases
§ Metabolic Disorders
§ Neuromuscular Disorders
§ Oncological Disorders
§ Ophthalmic Disorders
§ Other Therapeutic Areas
- Key Geographical Regions
§ North America
§ Europe
§ Asia-Pacific and the Rest of the World
To request sample pages, please visit https://www.rootsanalysis.com/reports/view_document/oligonucleotide-synthesis/304.html
Key Questions Answered
§ Who are the leading players offering oligonucleotide synthesis, modification and purification services for research, diagnostic and therapeutic applications?
§ What are the preferred modification and purification methods used for oligonucleotides?
§ What are the key challenges faced by stakeholders engaged in this domain?
§ What kind of partnership models are commonly adopted by industry stakeholders?
§ What are the recent expansion initiatives undertaken by service providers within this domain?
§ Which therapy developers are likely to partner with oligonucleotide manufacturing service providers?
§ What is the annual, clinical and commercial demand for oligonucleotides?
§ What is the current, installed manufacturing capacity for oligonucleotides?
§ What percentage of oligonucleotide manufacturing operations are outsourced to service providers?
§ What factors should be taken into consideration while deciding whether the manufacturing operations for oligonucleotides should be kept in-house or outsourced?
§ What are the different initiatives undertaken by big pharma players engaged in this domain, in the recent past?
§ What are the opportunities in emerging markets for oligonucleotide manufacturing?
§ How is the current and future market opportunity likely to be distributed across key market segments?
§ What are the anticipated future trends related to oligonucleotide manufacturing?
You may also be interested in the following titles:
1. Pharmaceutical Contract Manufacturing Market (3rd Edition), 2021-2030
2. Biopharmaceutical Contract Manufacturing Market (4th Edition), 2021-2030
3. Antisense Oligonucleotide Therapeutics Market, 2020-2030
4. RNAi Therapeutics Market (2nd Edition), 2019 - 2030
Contact Us:
Ben Johnson
+1 (415) 800 3415
+44 (122) 391 1091












