views

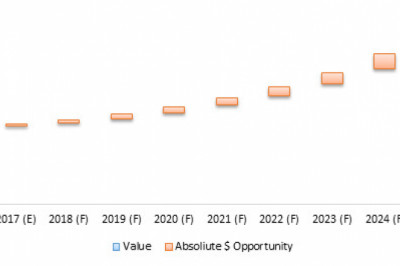
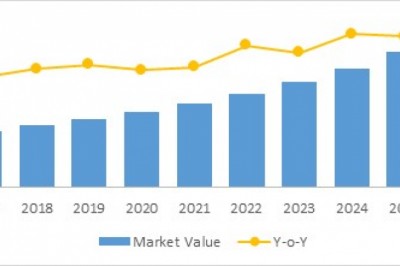
Transparency Market Research (TMR) has published a new report titled, ‘HIV/AIDS Immunoassay Diagnostics Market – Global Industry Analysis, Size, Share, Growth, Trends, and Forecast, 2019–2027.’ According to the report, the global HIV/AIDS immunoassay diagnostics market was valued at ~US$ 550 Mn in 2018 and is projected to expand at a CAGR of ~5% from 2019 to 2027.
Request Brochure –
https://www.transparencymarketresearch.com/sample/sample.php?flag=B&rep_id=74619
Overview
· Human immunodeficiency virus (HIV) belongs to the retrovirus family. HIV is transmitted through unprotected sex with an infected person, transfusion of HIV infected blood, and sharing needles contaminated with HIV infected blood.
· Rise in prevalence of HIV across the globe, HIV infections among young population, blood donations, awareness about HIV diagnosis across the globe, and favorable initiatives by governments for HIV/AIDS diagnosis and prevention are key factors that are anticipated to drive the global HIV/AIDS immunoassay diagnostics market during the forecast period
· North America held a major share of the global HIV/AIDS immunoassay diagnostics market in 2018. It is primarily driven by factors such as presence of key market players, highly structured health care industry, and key developments by market players such as product launch approvals, key mergers, and acquisitions.
· The HIV/AIDS immunoassay diagnostics market in Asia Pacific is expected to expand at a high CAGR of ~7% from 2019 to 2027. Increase in the number of people with sexually transmitted diseases, rise in incidence of HIV infection among young adults, product approvals, and rise in awareness about HIV/AIDS diseases are factors anticipated to fuel the growth of the HIV/AIDS immunoassay diagnostics market in the region.
TOC of Report – https://www.transparencymarketresearch.com/report-toc/74619
Increase in Prevalence of HIV Infections across the Globe to Drive Market
· HIV is basically transmitted through unprotected sex with an infected person, transfusion of HIV infected blood, and sharing needles contaminated with HIV infected blood. Rise in the number of patients with HIV contamination at an alarming rate in several countries is a major factor likely to drive the revenue of a large number of diagnostic labs and clinics in the near future.
· According to the WHO, HIV continues to be a major global public health issue. Based on UNAIDS report 2018, 39.6 million adults and children were living with HIV and approximately 940,000 individuals died from HIV globally.
· Approximately 1.7 million people were newly infected with HIV globally, in 2018
· Majority of the affected population lives in lower and middle-income countries. Middle East & Africa and Asia Pacific have high incidence rate of HIV infections across the globe.
· Hence, rise in prevalence of HIV is projected to increase the demand for HIV diagnostics which, in turn, is anticipated to boost the growth of the market
· According to HIV.org, approximately 1.1 million people in the U.S. were living with HIV in 2018
· According to Avert.org, approximately 2.2 million people in Western Europe were living with HIV infections in 2018. Moreover, Eastern Europe witnessed a 29% increase in new HIV infections annually between 2010 and 2018.
Request Customization on HIV/AIDS Immunoassay Diagnostics Market Report –
https://www.transparencymarketresearch.com/sample/sample.php?flag=CR&rep_id=74619
Kits & Reagents Dominated HIV/AIDS Immunoassay Diagnostics Market
· In terms of product, the global HIV/AIDS immunoassay diagnostics market can be classified into analyzers and kits & reagents
· The kits & reagents segment dominated the global HIV/AIDS immunoassay diagnostics market in 2018 and the trend is projected to continue during the forecast period. Several key market players are investing in the expansion of their assay offerings. Leading market players offer more than 100 tests across different application areas using the CLIA technology. Moreover, low cost and short test time are likely to propel the segment during the forecast period.
CLIA Technology to Lead Global HIV/AIDS Immunoassay Diagnostics Market
· In terms of technology, the global HIV/AIDS immunoassay diagnostics market has been segmented into enzyme-linked immunosorbent assay (ELISA), chemiluminescence immunoassay (CLIA), and others (immunofluorescent assay (IFA), radioimmunoassay (RIA), and enzyme-linked fluorescent assay (ELFA)). The chemiluminescence immunoassay (CLIA) segment accounted for majority of the market share in 2018. The segment is projected to register the highest market attractiveness index by 2027. High sensitivity of the CLIA technology and its ability to detect HIV-1 and HIV-2 antibodies and p24 antigen separately, which is useful in the early diagnosis of HIV infections, is anticipated to drive the segment. Moreover, availability of a wide range of assays and high throughput of CLIA analyzers are likely to propel the segment from 2019 to 2027.
· The ELISA segment is anticipated to expand at a rapid CAGR during the forecast period. ELISA is considered as the gold standard technique and a screening tool for HIV infections.
Diagnostic Laboratories to Flourish in Global HIV/AIDS Immunoassay Diagnostics Market
· In terms of end user, the global HIV/AIDS immunoassay diagnostics market has been categorized into hospitals, diagnostic laboratories, blood banks, and others
· The diagnostic laboratories segment held a major share of the global HIV/AIDS immunoassay diagnostics market in 2018. Growth of the segment can be attributed to the increase in incidence rate of HIV infections leading to high demand for early disease diagnosis. Moreover, high-volume consumption of immunoassay reagents and test kits such as ELISA and CLIA in diagnostic laboratories for the diagnosis of HIV infections is expected to boost the growth of the segment.
Buy HIV/AIDS Immunoassay Diagnostics Market Report –
https://www.transparencymarketresearch.com/checkout.php?rep_id=74619<ype=S
North America to Dominate Global Market; Asia Pacific to Offer Significant Incremental Opportunity
· In terms of region, the global HIV/AIDS immunoassay diagnostics market has been segmented into North America, Europe, Latin America, Asia Pacific, and Middle East & Africa. North America held a major share of the global HIV/AIDS immunoassay diagnostics market in 2018. It is primarily driven by factors such as presence of key market players, highly structured health care industry, and key development by market players such as product launch approvals, key mergers, and acquisitions.
· In May 2016, DiaSorin and Beckman Coulter signed an agreement for the promotion and distribution of DiaSorin’s hepatitis (A, B, and C) and HIV tests on DiaSorin’s Liaison XL platform in the U.S.
· In October 2017, Siemens Healthineers launched its new, integrated, automated immunoassay and chemistry analyzer ‘Atellica’. This move is estimated to attract more consumers toward complete laboratory automation and integration. Moreover, high awareness of HIV/AIDS disease among people is leading to the adoption of new therapy for diagnosis of HIV/AIDS. This factor is anticipated to drive the HIV/AIDS immunoassay diagnostics market in the region
· The HIV/AIDS immunoassay diagnostics market in Asia Pacific is projected to grow at a rapid pace in the near future. India, China, and Australia are expected to be lucrative markets, owing to setting up of new diagnostic laboratories and rise in awareness about the benefits of diagnosis of HIV using kits, reagents, and assays. For instance, in June 2017, Shenzhen New Industries Biomedical Engineering Co., Ltd. (SNIBE) received approval from the U.S. FDA for its automated immunoassay analyzer ‘Maglumi 2000’, becoming the first China-based manufacturer to receive this approval. This product approval is expected to enable the company to enhance its product portfolio and strengthen its market position in China.
Growth Strategies of Key Players
· Key players operating in the global HIV/AIDS immunoassay diagnostics market are F. Hoffmann-La Roche Ltd, Bio-Rad Laboratories, Inc., Siemens Healthineers AG, DiaSorin S.p.A., Abbott Laboratories, Ortho Clinical Diagnostics (a part of Carlyle Group), Shenzhen New Industries Biomedical Engineering Co., Ltd., bioMérieux SA, and Fujirebio (Miraca Group)
· These companies engage in research & development activities to develop novel products in order to expand product offerings and customer base. For instance, in June 2017, Shenzhen New Industries Biomedical Engineering Co., Ltd. (SNIBE) received approval from the U.S. FDA for its automated immunoassay analyzer ‘Maglumi 2000’, becoming the first China-based manufacturer to receive this approval. In June 2016, Ortho Clinical Diagnostics received the CE Mark approval for its Vitros HIV combo test, which can be used in its VITROS series of automated immunoassay analyzers. This has expanded the company’s portfolio in infectious disease assays.
HIV/AIDS Immunoassay Diagnostics Market: Segmentation
HIV/AIDS Immunoassay Diagnostics Market, by Product
· Analyzers
· Kits & Reagents
HIV/AIDS Immunoassay Diagnostics Market, by Technology
· Enzyme-linked Immunosorbent Assay (ELISA)
· Chemiluminescence Immunoassay (CLIA)
· Others
o Immunofluorescent Assay (IFA)
o Radioimmunoassay (RIA)
o Enzyme-linked Fluorescent Assay (ELFA)
HIV/AIDS Immunoassay Diagnostics Market, by End User
· Hospitals
· Diagnostic Laboratories
· Blood Banks
· Others
HIV/AIDS Immunoassay Diagnostics Market, by Region
· North America
o U.S.
o Canada
· Europe
o Germany
o France
o U.K.
o Italy
o Spain
· Asia Pacific
o China
o Japan
o India
o Australia & New Zealand
o Rest of Asia Pacific
· Latin America
o Brazil
o Mexico
o Rest of Latin America
· Middle East & Africa
o GCC
o South Africa
o Rest of Middle East & Africa
About Us
Transparency Market Research is a global market intelligence company, providing global business information reports and services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insight for thousands of decision makers. Our experienced team of analysts, researchers, and consultants, uses proprietary data sources and various tools and techniques to gather and analyze information.
Our data repository is continuously updated and revised by a team of research experts, so that it always reflects the latest trends and information. With a broad research and analysis capability, Transparency Market Research employs rigorous primary and secondary research techniques in developing distinctive data sets and research material for business reports.
Contact
Transparency Market Research,
90 State Street, Suite 700,
Albany, NY 12207
Tel: +1-518-618-1030
USA - Canada Toll Free: 866-552-3453
Email: sales@transparencymarketresearch.com
Website: https://www.transparencymarketres