views
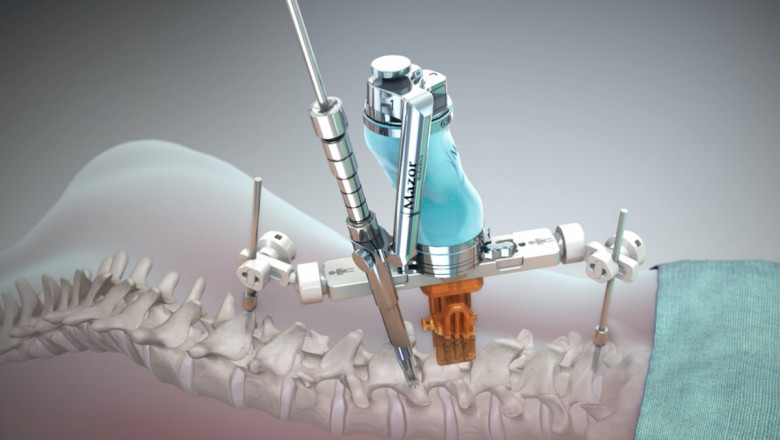
The worldwide Spine Surgery Devices Market is predicted to develop due to the frequent approval of innovative surgical devices used in spinal injuries by regulatory agencies. For example, the US Food and Drug Administration (FDA) granted Meditech Spine LLC licence to commercialise their Cure Opel – C system in September 2018. Cure Opel – C is a new technology that complements the company's current Cure ACP and Talos – C (HA) Interbody Systems. It has a one-of-a-kind lock mechanism that prevents screws from backing out once they've been installed in the spinal column.
In addition, Camber Spine Technologies, LLP gained FDA clearance for their product Siconus SI Fusion Screw System in January 2017. Larger bones, such as the sacrum, are supported and fixed by it. Camber Spine Technologies collaborated with the Institute for Musculoskeletal Science and Education to create this device. The worldwide Spine Surgery Devices Market is likely to increase as a result of regulatory approvals of innovative devices.
Source Link- https://cmi-latestreportorientedblogs.blogspot.com/2022/06/spine-surgery-devices-market-analysis.html












