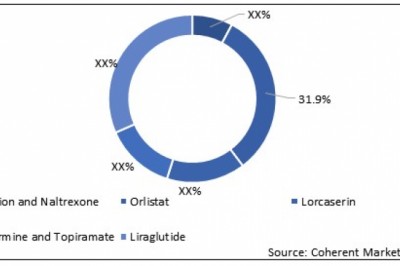
views
Although PCR is the standard test used for the detection of Covid-19, ICMR has broadened the range of options forCovid-19 test that includes Rapid CLIA ELISA kits. In India, this test forCOVID -19 has got approval only for surveys in the areas of high risk and some segments like the containment zones, some immune-compromised individuals along with the frontline and the health workers.
The Indian Council of Medical Research (ICMR) has recently approved those ELISA test kits for Covid-19 that have been developed by two companies. These test kits were the first ELISA test kits that got approval in addition to those that have been used by ICMR’ own technology thereby adding to the bucket of choices for COVID-19 testing. ELISA kits fall under the category of medical devices and have already been granted the appropriate medical equipment registration.
The ELISA test for Covid-19 in India has got approval only for serosurveys that gives an estimation of the proportion of the population who are exposed to infection and for all surveys that are present in the high-risk areas as well as segments like the containment zones, immunocompromised individual and some of the frontline health workers.Based on the level of the infection seroprevalence, matching the public health interventions can be executed for better prevention as well as disease control.
ICMR has approved several companies for the manufacture of Rapid CLIA ELISA KITS as on May 26, 2020. This includes Zydus Cadila, Meril Diagnostics, Voxtur Bio, Trivitron Healthcare, J Mitra & Co, Karwa Enterprise, and Avecon Healthcare. It is true that using Elisa for testing is not very expensive and is rapid but its use is still quite limited.
A person might test positive in those tests at various points of time during infection. After an infection, the percentage of viral load might be high in the respiratory tract of the person with in a few days but if the person has not made antibodies, this Elisa test might be negative. After a few days when the person produces antibodies Rapid CLIA Elisa test will show a positive result. The Rapid Elisa test shows how widespread the infection is.