views
RootsAnalysis is pleased to announce the publication of its recent study, titled,“Global Preventive Vaccines Market, 2020-2030”.
KeyInclusions
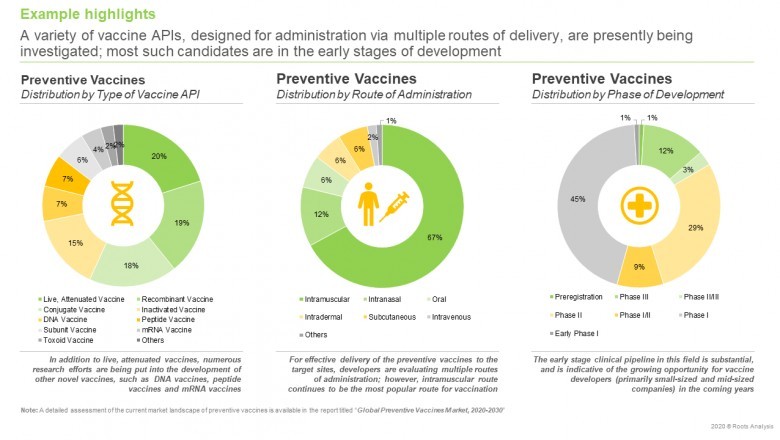
· A detailed assessment of the current marketlandscape, including information on type of developer (industry, non-industry,industry / non-industry), phase of development (phase I, phase I/II, phase II,phase II/III, phase III and preregistration) of lead candidates, route ofadministration (intramuscular, intranasal, oral, intradermal, subcutaneous,intravenous and others), type of vaccine API (live, attenuated vaccine,recombinant vaccine, conjugate vaccine, inactivated vaccine, DNA vaccine,peptide vaccine, subunit vaccine, mRNA vaccine, toxoid vaccine and others),dosage form (ready to use liquid, lyophilized powder, nasal spray, capsule andtablet), dosage (single dose, 2 doses, 3 doses, 4 doses, 5 doses and 6 doses),target disease indication and target patient population (children, adults andseniors).
· A competitiveness analysis of preventivevaccine developers, taking into consideration supplier strength (based oncompany size and its experience in this field) and pipeline strength (based onthe number of clinical-stage vaccine candidates, highest phase of development,number of compatible routes of administration, number of indications evaluatedand target patient population).
· Elaborate profiles of the key preventivevaccine developers (shortlisted based on a proprietary criterion) across NorthAmerica, Europe and Asia Pacific. Each profile includes a brief overview of thecompany, its year of establishment, location of headquarters, number ofemployees and financial information (if available). In addition to this, theprofile includes information on the various clinical-stage vaccine candidatesdeveloped by the company. Further, we have provided the recent developments ofthe company and an informed future outlook.
· A detailed analysis of more than 1,400completed, ongoing and planned clinical studies of preventive vaccines,highlighting prevalent trends across various relevant parameters, such as trialregistration year, phase of development, trial recruitment status, studydesign, trial focus area, type of preventive vaccine (based on pathogen),target disease indication(s), type ofsponsor / collaborator, leading industry sponsors / collaborators (in terms ofnumber of trials conducted), enrolled patients population and regionaldistribution.
· An overview of the ongoing vaccinedevelopment initiatives for complex conditions, such as COVID-19, Ebola virusdisease, HIV/AIDS, malaria and zika virus infection, including information ondisease, its global burden, current treatment landscape and preventive vaccineresearch landscape. Further, we have provided the information on investmentsmade and recent developments in the domain.
· An analysis of the investments made in thisdomain, during the period between 2015 and 2020 (till March), including seedfinancing, venture capital financing, debt financing, grants, capital raisedfrom IPOs and subsequent offerings, at various stages of development incompanies that are engaged in developing preventive vaccines.
· A case study on contract manufacturinglandscape for vaccines, featuring a comprehensive list of active CMOs andanalyses based on a number of parameters, such as year of establishment,company size, scale of operation (preclinical, clinical and commercial),geographical location, number of vaccine manufacturing facilities, types ofservices offered (cell / virus banking, analytical development / testing,formulation, process development, fill / finish and regulatory filings), typeof expression systems used for vaccine production (mammalian, microbial andothers) and type of vaccine manufactured.
Thereport also features the likely distribution of the current and forecastedopportunity across important market segments, mentioned below:
Routeof Administration
· Intramuscular
· Subcutaneous
· Oral
· Intravenous
· Others
Typeof Vaccine
· Pneumococcal Conjugate Vaccine
· Human Papilloma Virus Vaccine
· Rotavirus Vaccine
· Influenza Vaccine
· MMR Vaccine
· Tetanus and Diphtheria Booster Vaccine
· Varicella Vaccine
· DTaP-Hib-IPV Vaccine
· DTaP-HepB-Hib-IPV Vaccine
· Others
Typeof Vaccine API
· Live, Attenuated Vaccine
· Inactivated Vaccine
· Conjugate Vaccine
· Subunit Vaccine
· Toxoid Vaccine
· Others
TargetPatient Population
· Pediatric Patients
· Adults
KeyPlayers
· GlaxoSmithKline
· Merck
· Sanofi Pasteur
· Pfizer
· Emergent BioSolutions
· CSL
· Others
KeyGeographical Regions
· North America
· Europe
· Asia Pacific
· Rest of the World
Keycompanies covered in the report
· Bio Farma
· Emergent BioSolutions
· GC Pharma
· GlaxoSmithKline
· Janssen
· Merck
· Novavax
· Pfizer
· Sanofi Pasteur
· Valneva
Formore information, please click on the following link:
https://www.rootsanalysis.com/reports/view_document/preventive-vaccines/318.html
AboutRoots Analysis
RootsAnalysis is one of the fastest growing marketresearch companies, sharing fresh and independent perspectives in thebio-pharmaceutical industry. The in-depth research, analysis and insights aredriven by an experienced leadership team which has gained many years ofsignificant experience in this sector. If you’d like help with your growingbusiness needs, get in touch at info@rootsanalysis.com
ContactInformation
RootsAnalysis Private Limited
GauravChaudhary
+1(415) 800 3415
Gaurav.Chaudhary@rootsanalysis.com