views

[299 Pages Report] The global cell & gene therapy manufacturing services size is projected to reach USD 13.8 billion by 2026 from USD 7.7 billion in 2021, at a CAGR of 12.4% during the forecast period. The market is primarily driven by the prevalence of cancer and other target diseases. The development of therapies for key diseases, such as cancer, has attracted significant attention. Furthermore, increasing investments of pharmaceutical companies in the development of novel drugs is also fueling the market growth.
The pandemic slowed the economic growth of various countries, including the US, Germany, the UK, India, and China. It also resulted in control measures that impacted operations in life science organizations, such as production and research.
The impact varied due to changes in COVID-19 case volumes throughout the year, but the worst effect was seen in April 2020. Trials involving respiratory disease, oncology, ID/anti-infectives, and cardiovascular disease were the worst-hit during this time.
For More Info, Download PDF Brochure @ https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=180609441
The pharmaceutical industry, in particular, is R&D-intensive. Pharmaceutical companies invest in R&D to deliver high-quality and innovative products to the market. The trend suggests that the top pharma companies are increasing their R&D efficiencies through heavy R&D investments to see returns on their investment in the long run and through collaborative R&D efforts. According to an EvaluatePharma report, the worldwide pharmaceutical R&D spending was valued at USD 136 billion in 2012; this increased to USD 186 billion in 2019.
With the impact of COVID-19, the global pharma R&D growth rate has dropped to 0.3% from 2019–2020. As per report findings, this R&D spend is expected to grow steadily between 2019 and 2026 at a CAGR of 3.2% to reach USD 232.5 billion, slower than the historical CAGR of 4.6% between 2012 and 2019.
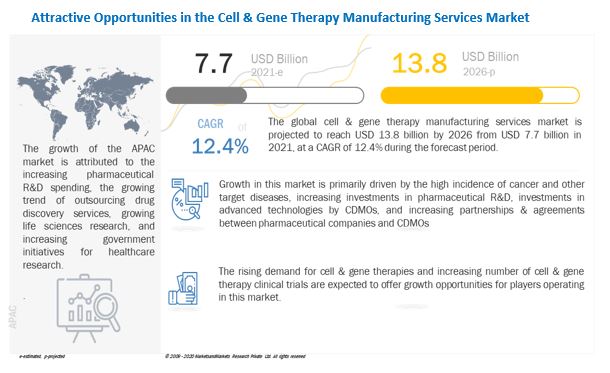
The increase in pharmaceutical R&D has resulted in a sharp increase in the number of cell & gene therapy candidates under development. This has made it necessary to outsource manufacturing services to develop cost-effective and efficient cell & gene therapies.
Currently, there are 1,200 cell & gene therapies in trials worldwide. There are more than 700 investigational cell & gene therapies in clinical development in the US alone. However, manufacturing facilities have not kept up. It has been estimated that hundreds of facilities will be needed to manufacture the treatments that are now in clinical trials.
According to a 2020 PhRMA report on the cell & gene therapy pipeline in 2018, there were 289 cell & gene therapies in clinical development by biopharmaceutical companies. This number increased by 25% in 2020, with 362 cell & gene therapies in clinical development. In addition to this, according to data released by CGT Catapult, there were 154 ATMP clinical trials ongoing in the UK in 2020 compared to the 127 trials reported in 2019, indicating an increase of more than 20%.
Key players in the cell & gene therapy manufacturing services market include Thermo Fisher Scientific (US), Merck KGaA (Germany), Charles River Laboratories (US), Lonza (Switzerland), Catalent (US), WuXi AppTec (China), Takara Bio Inc. (Japan), Nikon Corporation (Japan), FUJIFILM Holdings Corporation (Japan), F. Hoffmann-La Roche Ltd. (Switzerland), Oxford Biomedica plc (UK), and Cell and Gene Therapy Catapult (UK).












