views
Roots Analysis has done a detailed study on “Global Stem Cells Market: Focus on Clinical Therapies, 2020–2030 (Based on Source (Allogeneic, Autologous); Origin (Adult, Embryonic); Type (Hematopoietic, Mesenchymal, Progenitor); Lineage (Amniotic Fluid, Adipose Tissue, Bone Marrow, Cardiosphere, Chondrocytes, Corneal Tissue, Cord Blood, Dental Pulp, Neural Tissue Placenta, Peripheral Blood, Stromal Cells); and Potency (Multipotent, Pluripotent))”covering key aspects of the industry’s evolution and identifying potential future growth opportunities.
To order this 500+ page report, which features 185+ figures and 220+ tables, please visit this - https://www.rootsanalysis.com/reports/view_document/global-stem-cell-therapy-market-focus-on-cardiovascular-and-metabolic-disorders-2019-2030/246.html
Key Market Insights
§ Eminent representatives from different biopharmaceutical companies confirm the growing interest in stem cell therapy research, highlighting the prevalent and anticipated trends in ongoing R&D activities.
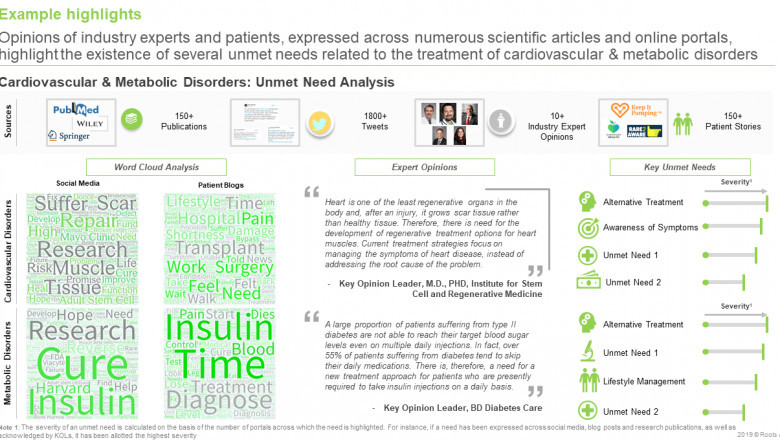
§ More than 120 industry players are engaged in evaluating the potential of over 280 stem cell therapy products for the treatment of a variety of disease indications.
§ The pipeline features a variety of marketed / clinical therapies being evaluated across different stages targeting various types of stem cells; these products are designed for administration via diverse routes of administration.
§ Stem cell therapies have demonstrated the applicability across over 25 unique therapeutic areas; cardiovascular, neurological, musculoskeletal and autoimmune / inflammatory disorders are presently the primary focus.
§ Several organizations have extended financial support to aid research efforts in this domain; the current focus is on the evaluation of different stem cell types across multiple clinical conditions.
§ In the last few years, over 540 clinical trials, evaluating various types of stem cell therapies in more than 52,000 patients, have been initiated; most of these trials are presently being conducted in centers across the US.
§ As several therapy candidates progress towards regulatory approval, developers are exploring diverse commercialization strategies to be implemented across different stages of a product’s launch cycle.
§ Owing to the rising demand for stem cell therapies, and challenges associated with manufacturing and scale up, industry players have demonstrated preferences to engage the services of contract service providers.
§ More than 80 industry / non-industry players, based in different regions across the globe, claim to provide contract development and manufacturing services to cater to the specific unmet needs of therapy developers.
§ Future growth of the market is likely to be driven by the success of different types of stem cell therapies, which are allogeneic and autologous in nature, as stakeholders continue to remain optimistic about proprietary products.
§ The foreseen future market opportunity, based on the revenue generation potential of such therapies, is anticipated to be distributed across different therapeutic areas, end-users and key geographical regions.
§ Products based on bone marrow derived adult stem cells are likely to continue to dominate the market in the mid-long term; adoption of therapies administered via novel routes of administration is likely to gradually rise.
For more information, please visit https://www.rootsanalysis.com/reports/view_document/global-stem-cell-therapy-market-focus-on-cardiovascular-and-metabolic-disorders-2019-2030/246.html
Table of Contents
1. PREFACE
1.1. Scope of the Report
1.2. Research Methodology
1.3. Chapter Outlines
2. EXECUTIVE SUMMARY
3. INTRODUCTION
3.1. Chapter Overview
3.2. Overview of Stem Cell Therapies
3.2.1. Historical Evolution of Stem Cell Research
3.2.2. Classification of Stem Cells
3.2.2.1. Based on Source of Stem Cell
3.2.2.2. Based on Origin of Stem Cell
3.2.2.3. Based on Potency of Stem Cell
3.2.3. Administration of Stem Cell Therapies
3.2.4. Applications of Stem Cell Therapies
3.2.5. Challenges Associated with Development of Stem Cell Therapies
3.3. Regulatory Guidelines for Stem Cell Therapies
3.3.1. Current Scenario
3.3.2. Regulatory Guidelines in North America
3.3.3. Regulatory Guidelines in Europe
3.3.4. Regulatory Guidelines in Japan
3.4. Prevalent Trends Related to Stem Cell Therapies
3.4.1. Emerging Focus Areas
3.4.2. Key Historical Trends
3.4.3. Geographical Activity
3.5. Growth Drivers and Roadblocks
4. MARKET LANDSCAPE
4.1. Chapter Overview
4.2. Stem Cell Therapies: Marketed and Development Pipeline
4.2.1. Analysis by Phase of Development
4.2.2. Analysis by Source of Stem Cell
4.2.3. Analysis by Origin of Stem Cell
4.2.4. Analysis by Type of Stem Cell
4.2.5. Analysis by Lineage of Stem Cell
4.2.6. Analysis by Potency of Stem Cell
4.2.7. Analysis by Target Indication
4.2.8. Analysis by Therapeutic Area
4.2.9. Analysis by Route of Administration
4.3. Stem Cell Therapies: Additional Information
4.3.1. Information on Dosage and Commercial Rights
4.3.2. Information on Designations Awarded
4.4. Stem Cell Therapies: List of Technology Platforms
4.5. Stem Cell Therapies: List of Therapy Developers
4.5.1. Analysis by Year of Establishment
4.5.2. Analysis by Company Size
4.5.3. Analysis by Location of Headquarters
4.5.4. Analysis by Company Size and Location of Headquarters
4.5.5. Leading Drug Developers: Analysis by Number of Stem Cell Therapies
4.5.6. Leading Drug Developers: 4D Bubble Analysis based on Strength of Pipeline, Target Indication and Company Size
4.6. Heptagon Representation: Analysis by Phase of Development and Key Therapeutic Area
4.7. Grid Representation: Analysis by Phase of Development, Source of Stem Cell and Therapeutic Area
4.8. Tree Map Representation: Analysis by Therapeutic Area and Size of the Company
4.9. World Map Representation: Analysis of Regional Activity
5. COMPANY PROFILES
5.1. Chapter Overview
5.2. Anterogen
5.2.1. Company Overview
5.2.2. Product Portfolio: Clinical-Stage Stem Cell Therapies
5.2.3. Recent Developments and Future Outlook
5.3. Athersys
5.3.1. Company Overview
5.3.2. Product Portfolio: Clinical-Stage Stem Cell Therapies
5.3.3. Recent Developments and Future Outlook
5.4. CHA Biotech
5.4.1. Company Overview
5.4.2. Product Portfolio: Clinical-Stage Stem Cell Therapies
5.4.3. Recent Developments and Future Outlook
5.5. Cytopeutics
5.5.1. Company Overview
5.5.2. Product Portfolio: Clinical-Stage Stem Cell Therapies
5.5.3. Recent Developments and Future Outlook
5.6. Hope Biosciences
5.6.1. Company Overview
5.6.2. Product Portfolio: Clinical-Stage Stem Cell Therapies
5.6.3. Recent Developments and Future Outlook
5.7. Mesoblast
5.7.1. Company Overview
5.7.2. Product Portfolio: Clinical-Stage Stem Cell Therapies
5.7.3. Recent Developments and Future Outlook
5.8. Pluristem Therapeutics
5.8.1. Company Overview
5.8.2. Product Portfolio: Clinical-Stage Stem Cell Therapies
5.8.3. Recent Developments and Future Outlook
5.9. Takeda Pharmaceutical
5.9.1. Company Overview
5.9.2. Product Portfolio: Clinical-Stage Stem Cell Therapies
5.9.3. Recent Developments and Future Outlook
5.10. TICEBA
5.10.1. Company Overview
5.10.2. Product Portfolio: Clinical-Stage Stem Cell Therapies
5.10.3. Recent Developments and Future Outlook
6. KEY THERAPEUTIC AREAS
6.1. Chapter Overview
6.2. Autoimmune / Inflammatory Disorders
6.2.1. Crohn’s Disease
6.2.1.1. Disease Description
6.2.1.2. Epidemiology
6.2.1.3. Current Treatment Options
6.2.1.4. Side Effects of Current Treatment Options
6.2.1.5. Stem Cell Therapies for Crohn’s Disease
6.2.2. Ulcerative Colitis
6.2.2.1. Disease Description
6.2.2.2. Epidemiology
6.2.2.3. Current Treatment Options
6.2.2.4. Side Effects of Current Treatment Options
6.2.2.5. Stem Cell Therapies for Ulcerative Colitis
Contact Details
Gaurav Chaudhary
+1 (415) 800 3415












