views
The COVID-19 pandemic had a moderately positive effect on the sales of diagnostics products for sepsis. The global spread of covid-19 and the emerging cases of sepsis among covid-19 patients are likely to increase the demand for rapid diagnosis, accelerating the utilization of instruments, reagents, & assay kits for detection of sepsis. Lockdowns resulting from the pandemic caused people to delay undergoing health checkups, affecting the number of tests performed and reagent sales.
The current COVID-19 pandemic has highlighted the risk faced by older adults, who are more susceptible to complications, including acute respiratory distress syndrome, usually due to pneumonia, which increases the risk of developing sepsis. Thus, increasing the need for early diagnosis of sepsis among patients with covid-19 infections.
The most common hospital-acquired infections (HAIs) are urinary tract infections, pneumonia, and sepsis. HAIs can lead to sepsis in immunocompromised patients, geriatric patients, and patients suffering from chronic illnesses. In the US, the incidence of sepsis among hospitalized patients is rising by 8.7% per year.
It is estimated that there are more than 1,000,000 incidences of sepsis among hospitalized patients annually in the nation {Source: CDC, 2018). With the rapid increase in HAIs worldwide, the demand for sepsis diagnostic products is also expected to rise in the coming years.
The cost of molecular diagnostic tests is between USD 300 and USD 3,000, which is very high compared to the cost of blood culture tests, priced as low as ~USD 28–35. Companies focus on developing automated diagnostic devices based on advanced technologies, such as molecular diagnostics, on detecting sepsis but due to limited budgets, government hospitals (especially in emerging nations) and academic research laboratories cannot afford such systems.
For More Info, Download PDF Brochure @ https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=92673155
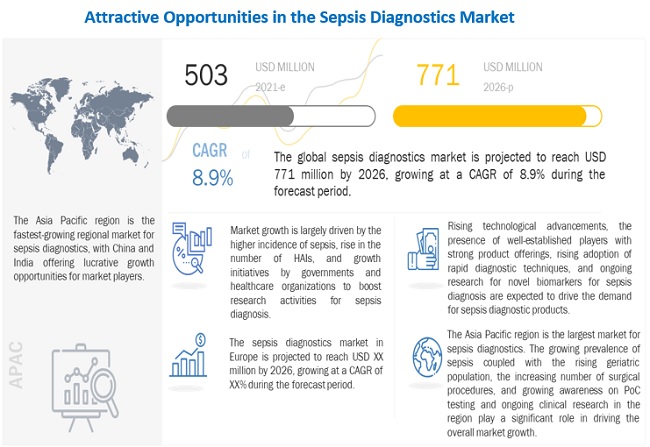
[236 Pages Report] The global sepsis diagnostics market size is expected to reach USD 771 million by 2026 from USD 503 million in 2021, at a CAGR of 8.9%. The demand for sepsis diagnostic products is expected to grow mainly due to factors such as the increasing public-private funding for sepsis diagnostic research activities, the growing burden of infectious diseases, the rising number of sepsis incidences, and growing government initiatives for creating sepsis awareness.
The sepsis diagnostics market, by product, the market is segmented into blood culture media, assays & reagent kits, instruments, and software. The blood culture media segment accounted for the largest share of market during the forecast period. This share can be attributed to the growing utilization of blood culture media by hospitals & pathology laboratories for the diagnosis of sepsis coupled with an increase in the availability of blood culture media in the market.
The prominent players operating in the global sepsis diagnostics market include bioMérieux (France), Becton, Dickinson and Company (US), Danaher Corporation (US), T2 Biosystems (US), Luminex (US), Roche Diagnostics (Switzerland), Thermo Fisher Scientific (US), Bruker Corporation (US), Abbott Laboratories (US), Immunexpress (Australia), Axis-Shield Diagnostics (UK), Quidel Corporation (US), Siemens Healthineers (Germany), EKF Diagnostics (UK), Seegene Inc., (South Korea), Boditech Med (South Korea), Alifax S.r.l. (Italy), AdvanDx (US), (US), Immunexpress (Australia), and Axis-Shield Diagnostics (UK).












