views

Non Invasive Prenatal Testing Industry Overview:
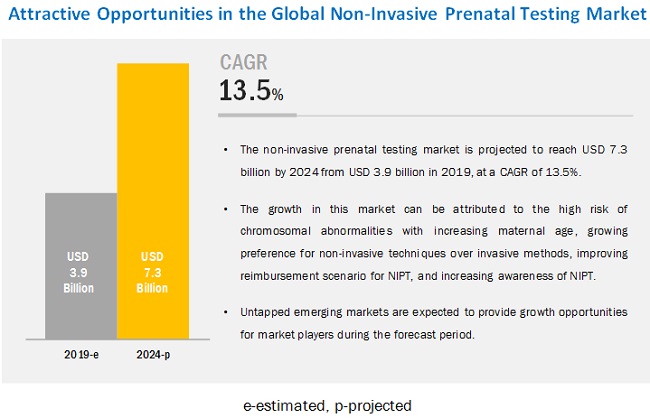
The global non-invasive prenatal testing market is estimated to reach USD 7.3 billion by 2024 from USD 3.9 billion in 2019, at a CAGR of 13.5% during the forecast period. Key factors driving the growth of this market include increasing preference for non-invasive techniques over invasive methods, increasing interest in reimbursement for NIPT, launch of new and advanced NIPT products, and increasing maternal age (increasing risk of chromosomal abnormalities). in baby)
Download PDF Brochure @ https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=145607690
NIPT tests can be performed in patients between 10 and 20 weeks of the gestation period. These tests require the extraction of fetal DNA from maternal blood to determine chromosomal abnormalities. The majority of cell-free DNA circulating in maternal blood is of maternal origin, and only 10–15% is of fetal origin. The amount of fetal fraction in the blood is affected by a number of factors.
The services segment is expected to grow at the highest CAGR during the forecast period.
On the basis of product and service, the NIPT market is segmented into products and services. The services segment is expected to grow at the highest CAGR during the forecast period.
The trisomy segment accounted for the largest share of the market
On the basis of application, the NIPT market is segmented into trisomy, microdeletion syndrome, and other applications. The trisomy segment accounted for the largest share of the non-invasive prenatal testing market.
North America dominated the NIPT market
Growth in the North American market is supported by the presence of a better reimbursement structure and a favorable funding scenario for research activities, rising awareness about NIPT, and the increasing demand for the early detection of birth defects in this region.
Some prominent players in the global Non Invasive Prenatal Testing market Include:
The prominent players in the market are Illumina, Inc. (US), Thermo Fisher Scientific Inc. (US), GE Healthcare (US), BGI (China), Agilent Technologies, Inc. (US), F. Hoffmann-La Roche Ltd. (Switzerland), PerkinElmer Inc. (US), Laboratory Corporation of America Holdings (US), Natera, Inc. (US), and Yourgene Health (UK)
Request for Sample Pages @ https://www.marketsandmarkets.com/requestsampleNew.asp?id=145607690
Non Invasive Prenatal Testing Market Segmentation
MarketsandMarkets has segmented the Non Invasive Prenatal Testing Market based on Product Type, End User application, and region:
Non-invasive Prenatal Testing Market, by Product
- Products
- Consumables
- Assay Kits & Reagents
- Disposables
- Instruments
- NGS Systems
- PCR Instruments
- Microarrays
- Ultrasound Devices
- Other Instruments
- Services
Non-invasive Prenatal Testing Products Market, by Method
- Ultrasound Detection
- Biochemical Screening Tests
- Cell-free DNA in Maternal Plasma Tests
Non-invasive Prenatal Testing Market, by Application
- Trisomy
- Microdeletion Syndrome
- Other Applications
Non-invasive Prenatal Testing Products Market, by End User
- Diagnostic Laboratories
- Hospitals
Non-invasive Prenatal Testing Market, by Region
- North America
- US
- Canada
- Europe
- Germany
- France
- UK
- Rest of Europe (RoE)
- Asia
- Rest of the World (RoW)












