views
Global PediatricClinical Trials Market, by Clinical Trial Phase (Pre-clinical, Phase I,Phase II, and Phase III), by Study Design (Interventional (Randomized Trial,Non-randomized Trial, and Crossover Trial), and Observational), by MedicalCondition (Neuropsychiatric Conditions, Infectious Diseases, Maternal andPerinatal Conditions, Respiratory Diseases, Cardiovascular Diseases, Cancer,Digestive Diseases, Diabetes, Nutritional Deficiencies, and Others), and byRegion (North America, Latin America, Europe, Asia Pacific, Middle East, andAfrica), is estimated to be valued at US$ 12,571.7 million in 2020 and isexpected to exhibit a CAGR of 9.0% over the forecast period (2020-2027), ashighlighted in a new report published by Coherent Market Insights.
Major companies (and morerecently medium sized companies) are actively entering into strategicpartnerships and collaborations with a limited number of CROs to increaseefficiency. The CRO industry and in particular, large CROs with globalcapabilities, considerable scientific knowledge, and expertise are often ableto perform the needed services with greater focus and at a lower cost than theclient could perform internally. For instance, in April 2017, Charles Riverentered into a strategic collaboration with Nimbus Therapeutics aimed atadvancing new programs in immunology, metabolic disorders, and oncology fromthe discovery phase through to Investigational New Drug (IND) submission. InOctober 2018, the pharmaceuticals division of Roche selected IQVIA’s commercialtechnology suite for deployment across more than 100 markets.
Moreover, in February 2020, WPDPharmaceuticals Inc. entered into a collaboration agreement with CNSPharmaceuticals, Inc., a biotechnology company specializing in the developmentof novel treatments for primary and metastatic brain and central nervous systemtumors, to initiate a Phase I clinical trial for Berubicin in pediatric braincancer, in Poland. This trend is likely to be followed by mid-size pharma andbiotech companies during the forecast period
Global Pediatric Clinical TrialsMarket – Impact of Coronavirus (Covid-19) Pandemic
The COVID-19 pandemic hasdrastically affected clinical trials. Many trials have paused enrollment and researchersare facing multiple challenges associated with setting up remote visits, andperforming laboratory and other study assessments.
According to a survey conductedby Medidata Solutions, Inc. (an American technology company, which develops andmarkets software as a service for clinical trials), on April 23, 2020, 63% ofsurvey respondents reported that they stopped recruiting new patients forongoing clinical trials and 43% of the respondents have postponed their studies
* The sample copy includes: Report Summary, Table ofContents, Segmentation, Competitive Landscape, Report Structure, Methodology.
Request a sample copyof this report: https://www.coherentmarketinsights.com/insight/request-sample/397
The following factors areconsidered for pediatric clinical drug trials:
Enrollment of patients who aremore susceptible to severe COVID-19 infections such as pediatric oncologypatients, have been restricted
Patients who are suffering frominfections, including those who have COVID-19 symptoms or were exposed toCOVID-19 suffering people, are excluded from participation in pediatric studiesassociated with immunosuppressive therapies
Due to the lockdown, patients areunable to come to the site for final phases of ongoing pediatric studies,hence, they have to be kept in the study and remain on the study drug for alonger duration than expected. Therefore, safety must be followed and evaluatedfor patients who are receiving prolonged treatment, as there may be positive ornegative effect of the investigational drugs on the patients.
Browse 36 Market Data Tables and37 Figures spread through 313 sPages and in-depth TOC on “Global PediatricClinical Trials Market, by Clinical Trial Phase (Pre-clinical, Phase I, PhaseII, and Phase III), by Study Design (Interventional (Randomized Trial,Non-randomized Trial, and Crossover Trial), and Observational), by MedicalCondition (Neuropsychiatric Conditions, Infectious Diseases, Maternal andPerinatal Conditions, Respiratory Diseases, Cardiovascular Diseases, Cancer,Digestive Diseases, Diabetes, Nutritional Deficiencies, and Others), and byRegion (North America, Latin America, Europe, Asia Pacific, Middle East, andAfrica).
The market is expected to gainsignificant traction during the forecast period, as various contract researchorganizations offer advanced pharmaco metric modeling and clinical trialsimulation technologies, juvenile formulation, and toxicology services acrosspediatric indications. Players in the clinical research industry are engaged inadoption of inorganic growth strategies to expand their capabilities to conductpre-clinical studies. For instance, in March 2020, Altasciences, a CRO in theU.S. and Canada engaged in early stage drug development, entered into apartnership agreement with Amador Bioscience, a CRO in China and the U.S., toexpand and promote drug development processes such as preclinical studies andto conduct early-stage clinical programs in China and North America.
Browse ResearchReport: https://www.coherentmarketinsights.com/market-insight/pediatric-clinical-trials-market-397
Key Takeaways of the GlobalPediatric Clinical Trials Market:
The global pediatric clinicaltrials market is expected to exhibit a CAGR of 9.0% over the forecast period,owing to robust product pipeline and increasing approval of new pediatric drugs
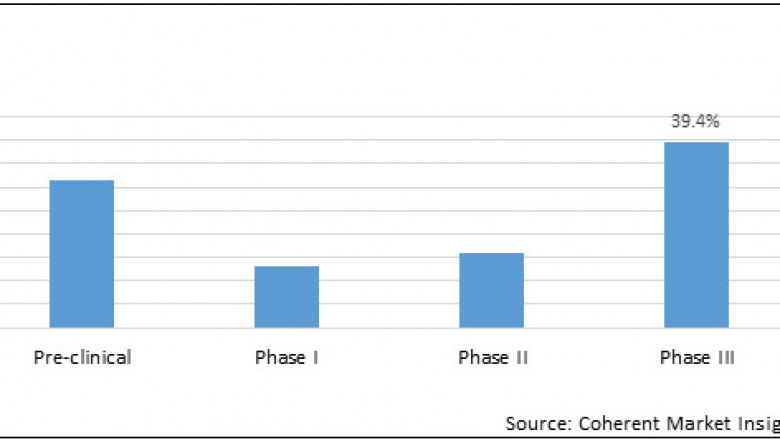
Among clinical trial phase, thepre-clinical segment is expected to hold a dominant position in the pediatricclinical trials market in 2020, owing to safety and efficacy concerns regardingnew molecules or drugs, as every new molecule must go through pre-clinicalstudy
Among study design type,randomized trial segment is expected to hold a dominant position in thepediatric clinical trials market in 2020, as randomized trial study designeliminates the chance of bias in clinical trials and is considered as the mostfavorable study design
Among medical condition,neuropsychiatric conditions segment is expected to hold dominant position inthe pediatric clinical trials market in 2020, owing to increasing number ofmental disorders in children and adolescents. Mental disorders mainly affectchildren before the age of 14 years.
Key players operating in theglobal pediatric clinical trials market include Syneos Health Inc., IQVIAHoldings, Inc., Charles River Laboratories International Inc., Covance Inc.,ICON plc, Pharmaceutical Product Development, LLC, Genentech (F. Hoffmann-LaRoche AG), Pfizer, Inc., Bristol - Myers Squibb, GlaxoSmithKline plc., SanofiS.A., Novartis AG, Johnson & Johnson, Merck & Co., Inc., TakedaPharmaceutical Company Limited, and Vertex Pharmaceuticals Inc.
Buy-Now this researchreport: https://www.coherentmarketinsights.com/insight/buy-now/397
AboutCoherent Market Insights:
CoherentMarket Insights is a prominent market research and consulting firm offeringaction-ready syndicated research reports, custom market analysis, consultingservices, and competitive analysis through various recommendations related toemerging market trends, technologies, and potential absolute dollaropportunity.
ContactUs:
mailto:sales@coherentmarketinsights.com
U.S.Office:
Name: Mr. Shah
CoherentMarket Insights 1001 4th Ave,
# 3200Seattle, WA 98154, U.S.
US : +1-206-701-6702
UK : +44-020-8133-4027
JAPAN : +050-5539-1737