views

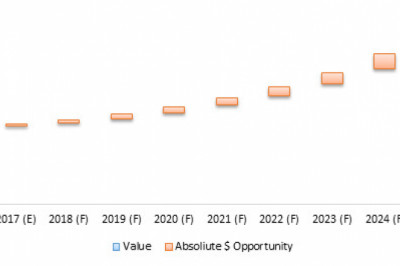
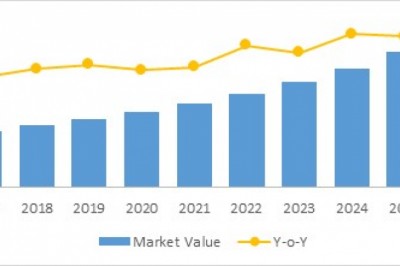
The Global ENTDisorder Treatment Market, by Treatment Type (Devices (Hearing Aid Devices,Voice Prosthesis, Nasal Splints, Hearing Implants and Others) and Drug Type(Antibiotics, Antihistamines, Steroids, Anti-inflammatory Drugs, Others)), ByOrgan Type (Ear, Nose, and Throat), by End User (Hospitals, Clinics, AmbulatorySurgical Centers, Home Care Settings, and Others), and by Region (NorthAmerica, Latin America, Europe, Asia Pacific, the Middle East, and Africa) wasvalued at US$ 9,521.1 million in 2016 and is projected to exhibit a CAGR of3.2% over the forecast period (2017–2025).
The ENT Disorders treatmentmarket is expected to gain significant traction with increasing incidence ofENT disorders and increasing environmental pollution globally. According to anarticle published by Harvard Medical School and Teaching Hospital, 2017, over360 million people across the globe are suffering from hearing loss and overhalf of this population can be treated with proper medication and care.
Development novel treatments forENT disorders through extensive R&D is expected to be a major factordriving the global ENT disorder treatment market growth Manufacturers arefocusing on development new and innovative treatments and therapies for ENTdisorders for both adult and pediatric segments, in order to maintain theirmarket share. For instance, Otonomy, Inc. — a U.S. based drug manufacturer —received FDA approval for OTIPRIO (ciproflaxin otic suspension) in December2015, for treatment of pediatric patients suffering with bilateral otitis mediawith effusion. Furthermore, in June 2017, Inspire Medical Systems received FDAapproval for Inspire 3028 neurostimulator for the treatment of obstructivesleep apnea.
* The sample copy includes: Report Summary, Table ofContents, Segmentation, Competitive Landscape, Report Structure, Methodology.
Request a sample copyof this report: https://www.coherentmarketinsights.com/insight/request-sample/1326
In May 2012, Meda Pharmaceuticalsreceived FDA approval for dymista (azelastine hydrochloride and fluticasonepropionate) for the treatment of seasonal allergic rhinitis that can beconsumed by intranasal administration or sprayed suspension.
Key players are continuouslyfocusing on developing and updating their products with new technologies totreat various types of disorders. Market players are conducting clinical trialsand a large number of treatment drugs and devices are in pipeline forapprovals. For instance, in February 2018, Tusker Medical announced their firstpatient enrollment for pivotal clinical trial for tympanostomy tube replacementthat is performed for recurrent ear infections or persistent fluid in themiddle ear. Browse 42 Market Data Tables and 36 Figures spread through 161Pages and in-depth TOC on “Global ENT Disorder Treatment Market”- by TreatmentType (Devices (Hearing Aid Devices, Voice Prosthesis, Nasal Splints, HearingImplants and Others) and Drug Type(Antibiotics, Antihistamines, Steroids,Anti-inflammatory Drugs, Others)), By Organ Type (Ear, Nose and Throat), by EndUser (Hospitals, Clinics, Ambulatory Surgical Centers, Home Care Settings andOthers), and by Region (North America, Latin America, Europe, Asia Pacific, theMiddle East, and Africa) - Global Forecast to 2025
Key players are focusing onstrategic merger and acquisition strategies for development andcommercialization of novel ENT therapeutics and medical devices. For instance,December 2017, Entellus Medical, Inc. — a leader in ENT drugs segment — entereda definitive merger agreement with Stryker Corporation for development ofeffective ENT solutions through collaboration. Moreover, in in July 2017,Entellus Medical acquired Spirox, Inc. a privately held company thatmanufactures, develops and markets ENT medical devices.
Browse ResearchReport: https://www.coherentmarketinsights.com/market-insight/ent-disorder-treatment-market-1326
Key Takeaways of the ENT DisorderTreatment Market:
The global ENT disorder treatmentmarket is expected to exhibit a CAGR of 3.22% over the forecast period, owingto rising incidence of ENT disorders coupled with deteriorating environmentalfactors such as rising air and noise pollution across the globe. For instance,according to CDC 2016, 30 million people in the U.S. were exposed to extremelyhigh decibels sound levels, at workplace, leading to hearing disorders.
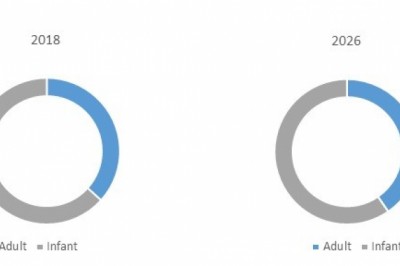
Among treatment type segment,drug segment holds a dominant position in the global ENT disorder treatmentmarket. This is owing to drug being the first line of treatment in most of theENT disorders.
Among end users, hospital segmentholds a dominant position in the global ENT disorder treatment market, as mostof the surgical, non-surgical as well as consultation procedures are conductedat hospitals.
Some of the major playersoperating in the global ENT disorder treatment market include Sanofi,AstraZeneca plc, Novartis AG, Pfizer Inc., Mylan N.V., Teva PharmaceuticalIndustries Ltd., GlaxoSmithKline Pharmaceuticals, Otonomy Inc., Merck &Co., Dr. Reddys Laboratories Ltd., Allergan plc, Cochlear Ltd., Sonova HoldingAG, Siemens Healthcare, Starkey Laboratories Inc., William Demant Holding A/S,Widex A/S, GN ReSound A/S, Sonic Innovations Inc., Panasonic Corp., Beltone,Rexton Inc., Avada Hearing Care, Miracle-Ear Inc., MED-EL GmbH, Nuear HearingAids Inc., Audiosync Inc., Bernafon, American Hearing Systems Inc., UnitronHearing Inc., and Zounds Inc.
Buy-Now this researchreport: https://www.coherentmarketinsights.com/insight/buy-now/1326
AboutCoherent Market Insights:
CoherentMarket Insights is a prominent market research and consulting firm offeringaction-ready syndicated research reports, custom market analysis, consultingservices, and competitive analysis through various recommendations related toemerging market trends, technologies, and potential absolute dollaropportunity.
ContactUs:
mailto:sales@coherentmarketinsights.com
U.S.Office:
Name: Mr. Shah
CoherentMarket Insights 1001 4th Ave,
# 3200Seattle, WA 98154, U.S.
US : +1-206-701-6702
UK : +44-020-8133-4027
JAPAN: +050-5539-1737